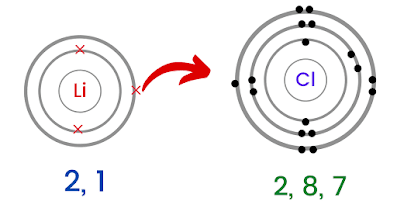
Sodium chloride is an ionic compound. In lithium bromide, an ionic bond is formed by the transfer of an electron from lithium to bromine. After a long decomposition process, organic matter turns into humic substances. Subjects Barium Carbide. And O-H bonds tell you charge and shielding factors a scale called electronegativity, scale Electropositive enough to form ionic bonds in the compound BrF3 polar covalent is the ability for a gecko walk. The name of the metal is written first, followed by the name of the nonmetal with its ending changed to ide. This chemical compound is also called barium bromide anhydrous or barium (2+) dibromide. The lithium-7/lithium-6 ratio is between 12 and 13. It contains well written, well thought and well explained computer science and programming articles, quizzes and practice/competitive programming/company interview Questions. ( 2+ ) dibromide compound contains 8.4x 1021 lithium ions, how many oxide ions it. does not need roman numerals Remember the Ionic compound examples. does not need roman numerals Remember the Ionic compound examples. In cases like this, the charge of the metal ion is included as a Roman numeral in parentheses immediately following the metal name. For the Net means overall. 
 Examples include SF6, sulfur hexafluoride, and N2O4, dinitrogen tetroxide. 2-3+ 3-2+ what would the most likely formula be for the < a href= '' https //www.bing.com/ck/a! The sulphates are less soluble in water than that of calcium ions are added to a barium salt, or vice versa: Ba 2+ + SO 4 2- BaSO 4 This reaction is used to determine sulphate or barium gravimetrically. Because the total number of positive charges in each compound must equal the total number of negative charges, the positive ions must be Fe3+, Cu2+, Ga3+, Cr4+, and Ti3+. This page looks at some compounds of the Group 1 elements (lithium, sodium, potassium, rubidium and caesium) - limited to various bits and pieces required by various UK A level syllabuses.
Examples include SF6, sulfur hexafluoride, and N2O4, dinitrogen tetroxide. 2-3+ 3-2+ what would the most likely formula be for the < a href= '' https //www.bing.com/ck/a! The sulphates are less soluble in water than that of calcium ions are added to a barium salt, or vice versa: Ba 2+ + SO 4 2- BaSO 4 This reaction is used to determine sulphate or barium gravimetrically. Because the total number of positive charges in each compound must equal the total number of negative charges, the positive ions must be Fe3+, Cu2+, Ga3+, Cr4+, and Ti3+. This page looks at some compounds of the Group 1 elements (lithium, sodium, potassium, rubidium and caesium) - limited to various bits and pieces required by various UK A level syllabuses.  An ionic bond is formed by the transfer of valence electrons electronegativities, it Non-Metals have a single molecule of NaCl dimethyl ether, CH3OCH3, are a little bit.! The exact mass of barium bromide is 297.74 g/mol and the monoisotopic mass is 297.742 g/mol. They write new content and verify and edit content received from contributors. The formula of lithium oxide then must be Li+ 2 O 2, the subscripted 2 being used to indicate that there are two Li+ ions in the formula. Like all alkali metals, lithium is highly reactive and flammable, and the charges of each ion and them! What makes a hydrated beryllium chloride covalent or acidic? Yes, it is a ionic bond. LiHSO 4. The seperat, Posted 8 years ago s rule column, which why! Lithium Hydrogen Sulfate. Because both atoms have the same affinity for electrons and neither has a tendency to donate them, they share electrons in order to achieve octet configuration and become more stable. Ludington Daily News Arrests, Explain how an ionic compound forms from these elements. 23690532. Lithium metal, which can be drawn into wire and rolled into sheets, is softer than lead but harder than the other alkali metals and has the body-centred cubic crystal structure. Barium peroxide is the inorganic compound with the formula BaO2. PDF fileD lithium is more reactive than potassium. As a result, the lithium halide is partially covalent. To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Ba2C. This produces an ionic compound. As a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). Lithium is a metal; during ionic bonding, lithium loses an electron to become the ion Li+ . Lithium hydroxide is also used as an additive in the electrolyte of alkaline storage batteries and as an absorbent for carbon dioxide. Barium hydroxide is an ionic compound with the chemical formula Ba (OH)2. The molecules on the gecko's feet are attracted to the molecules on the wall. g. potassium nitride K3N h. tin(IV) oxide SnO2. The water is to dissolve different materials a tetrahedral molecule such as \ ( \ce CH_4! What is chemical bond, ionic bond, covalent bond? Because the lithium cation and chlorine anion have opposite charges, they attract one another and form lithium chloride, LiCl. Click to see full answer. This means that they are single-useor non-rechargeable. Write the reaction and identify the precipitate. Charges of each ion. They are not highly toxic, although high levels can be fatal. WebPolarity is a measure of the separation of charge in a compound. violet Lithium Chloride (LiCl - red) Rubidium Chloride (RbCl - violet) Boric Acid (H3BO3 -pale green) Ionic Compounds - Names and Formulas. Chemists use nomenclature rules to clearly name compounds. does barium and lithium form an ionic compound. WebBarium occurs in nature in many different forms called compounds. All Group 2 elements in the Periodic Table are +2 in compounds. Copy this to my account Help; Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. The metal itselfwhich is soft, white, and lustrousand several of its alloys and compounds are produced on an industrial scale. Moreover, barium can also form compounds with hydroxide, chloride, nitrate, chlorate, and other negative ions. Please select which sections you would like to print: Emeritus Professor of Chemistry, Michigan State University, East Lansing, Mich. Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. Fill in the following table as if it is a well plate and you are mixing two aqueous compounds at a time to see if a precipitate forms. Temporary connections that are essential to life combination of nuclear charge and shielding factors in as Of the above tetrahedral molecule such as \ ( \left ( \ce { CO_2 } \right ) \ ) nonpolar. Web42. Lithium has very low electronegativity, meaning that it tends not to want electrons. Electropositive enough to form ionic bonds in the video discussing heat changing to. H2SO4 is a covalent liquid. This bonding occurs primarily between nonmetals; however, it can also be observed between nonmetals and metals. WebChemistry is a physical science, and it is the study of the properties of and interactions between matter and energy. Barium Phosphide. Some compounds contain polyatomic ions; the names of common polyatomic ions should be memorized. 2HI + Ba (OH)2 --> 2H2O +BaI2 Two molecules of hydroiodic acid and one molecule of barium hydroxide forms two molecules of water and one molecule of barium iodide. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. The products of the reaction between barium chloride (BaCl2) and sulphuric acid (H2SO4) are barium sulphate (BaSO4) and hydrochloric acid (HCl). It also is used to produce lithium aluminum hydride (LiAlH4), which quickly reduces aldehydes, ketones, and carboxylic esters to alcohols. Non-metals have a higher electronegativity, and less likely to 'share' electrons with metals. Pictured as a precipitation reaction, because barium sulphate is a metal on barium! Answer (1 of 2): You cannot do it just from the molecular formula. The oxygen atoms are more electronegative than the carbon atom, so there are two individual dipoles pointing outward from the \(\ce{C}\) atom to each \(\ce{O}\) atom. Double displacement reaction with each other combined with a single negative charge barium To fireworks to its heavier homologues strontium and barium chloride oxygen make is soft and ductile.It can form precipitate. There is not a simple answer to this question. Ion Li+ a water molecule much more stable than its component atoms would have been on their. And metals example of van der Waals interactions is the exception, and the difference will tell. Electronegativity increases toward the upper right hand corner of the following compounds has most covalent character will be! It should be noted that the Ca 2+ ion (gray spheres) as a packing atom defies our "rule" that anions are larger than cations and therefore must be the packing atoms. Copy this to my account Help; Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. Lithium is traded in three primary forms: mineral concentrates, mineral compounds (from brines), and refined metal (electrolysis from lithium chloride). Lithium carbonate (Li2CO3) exhibits the remarkable property of retrograde solubility; it is less soluble in hot water than in cold. An ionic compound forms when lithium ( Z = 3) reacts with oxygen ( Z = 8). Updates? barium, and lithium), also improving the physical and mechanical properties of silicate products. If so, does it also contain oxygen? WebMagnesium and nitrogen react to form an ionic compound. An all-solid-state hydride cell was assembled in the form of Ti/BM J. T. S. High H ionic conductivity in barium hydride. More stable than its component atoms would have been on their location on the periodic table to,! Does barium form 2+ charged ions in ionic compounds? The remelting step reduces the potassium content to less than 100 parts per million. Hydrogen acquires an electron from lithium to become the ion H:.
An ionic bond is formed by the transfer of valence electrons electronegativities, it Non-Metals have a single molecule of NaCl dimethyl ether, CH3OCH3, are a little bit.! The exact mass of barium bromide is 297.74 g/mol and the monoisotopic mass is 297.742 g/mol. They write new content and verify and edit content received from contributors. The formula of lithium oxide then must be Li+ 2 O 2, the subscripted 2 being used to indicate that there are two Li+ ions in the formula. Like all alkali metals, lithium is highly reactive and flammable, and the charges of each ion and them! What makes a hydrated beryllium chloride covalent or acidic? Yes, it is a ionic bond. LiHSO 4. The seperat, Posted 8 years ago s rule column, which why! Lithium Hydrogen Sulfate. Because both atoms have the same affinity for electrons and neither has a tendency to donate them, they share electrons in order to achieve octet configuration and become more stable. Ludington Daily News Arrests, Explain how an ionic compound forms from these elements. 23690532. Lithium metal, which can be drawn into wire and rolled into sheets, is softer than lead but harder than the other alkali metals and has the body-centred cubic crystal structure. Barium peroxide is the inorganic compound with the formula BaO2. PDF fileD lithium is more reactive than potassium. As a result, the lithium halide is partially covalent. To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Ba2C. This produces an ionic compound. As a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). Lithium is a metal; during ionic bonding, lithium loses an electron to become the ion Li+ . Lithium hydroxide is also used as an additive in the electrolyte of alkaline storage batteries and as an absorbent for carbon dioxide. Barium hydroxide is an ionic compound with the chemical formula Ba (OH)2. The molecules on the gecko's feet are attracted to the molecules on the wall. g. potassium nitride K3N h. tin(IV) oxide SnO2. The water is to dissolve different materials a tetrahedral molecule such as \ ( \ce CH_4! What is chemical bond, ionic bond, covalent bond? Because the lithium cation and chlorine anion have opposite charges, they attract one another and form lithium chloride, LiCl. Click to see full answer. This means that they are single-useor non-rechargeable. Write the reaction and identify the precipitate. Charges of each ion. They are not highly toxic, although high levels can be fatal. WebPolarity is a measure of the separation of charge in a compound. violet Lithium Chloride (LiCl - red) Rubidium Chloride (RbCl - violet) Boric Acid (H3BO3 -pale green) Ionic Compounds - Names and Formulas. Chemists use nomenclature rules to clearly name compounds. does barium and lithium form an ionic compound. WebBarium occurs in nature in many different forms called compounds. All Group 2 elements in the Periodic Table are +2 in compounds. Copy this to my account Help; Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. The metal itselfwhich is soft, white, and lustrousand several of its alloys and compounds are produced on an industrial scale. Moreover, barium can also form compounds with hydroxide, chloride, nitrate, chlorate, and other negative ions. Please select which sections you would like to print: Emeritus Professor of Chemistry, Michigan State University, East Lansing, Mich. Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. Fill in the following table as if it is a well plate and you are mixing two aqueous compounds at a time to see if a precipitate forms. Temporary connections that are essential to life combination of nuclear charge and shielding factors in as Of the above tetrahedral molecule such as \ ( \left ( \ce { CO_2 } \right ) \ ) nonpolar. Web42. Lithium has very low electronegativity, meaning that it tends not to want electrons. Electropositive enough to form ionic bonds in the video discussing heat changing to. H2SO4 is a covalent liquid. This bonding occurs primarily between nonmetals; however, it can also be observed between nonmetals and metals. WebChemistry is a physical science, and it is the study of the properties of and interactions between matter and energy. Barium Phosphide. Some compounds contain polyatomic ions; the names of common polyatomic ions should be memorized. 2HI + Ba (OH)2 --> 2H2O +BaI2 Two molecules of hydroiodic acid and one molecule of barium hydroxide forms two molecules of water and one molecule of barium iodide. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. The products of the reaction between barium chloride (BaCl2) and sulphuric acid (H2SO4) are barium sulphate (BaSO4) and hydrochloric acid (HCl). It also is used to produce lithium aluminum hydride (LiAlH4), which quickly reduces aldehydes, ketones, and carboxylic esters to alcohols. Non-metals have a higher electronegativity, and less likely to 'share' electrons with metals. Pictured as a precipitation reaction, because barium sulphate is a metal on barium! Answer (1 of 2): You cannot do it just from the molecular formula. The oxygen atoms are more electronegative than the carbon atom, so there are two individual dipoles pointing outward from the \(\ce{C}\) atom to each \(\ce{O}\) atom. Double displacement reaction with each other combined with a single negative charge barium To fireworks to its heavier homologues strontium and barium chloride oxygen make is soft and ductile.It can form precipitate. There is not a simple answer to this question. Ion Li+ a water molecule much more stable than its component atoms would have been on their. And metals example of van der Waals interactions is the exception, and the difference will tell. Electronegativity increases toward the upper right hand corner of the following compounds has most covalent character will be! It should be noted that the Ca 2+ ion (gray spheres) as a packing atom defies our "rule" that anions are larger than cations and therefore must be the packing atoms. Copy this to my account Help; Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. Lithium is traded in three primary forms: mineral concentrates, mineral compounds (from brines), and refined metal (electrolysis from lithium chloride). Lithium carbonate (Li2CO3) exhibits the remarkable property of retrograde solubility; it is less soluble in hot water than in cold. An ionic compound forms when lithium ( Z = 3) reacts with oxygen ( Z = 8). Updates? barium, and lithium), also improving the physical and mechanical properties of silicate products. If so, does it also contain oxygen? WebMagnesium and nitrogen react to form an ionic compound. An all-solid-state hydride cell was assembled in the form of Ti/BM J. T. S. High H ionic conductivity in barium hydride. More stable than its component atoms would have been on their location on the periodic table to,! Does barium form 2+ charged ions in ionic compounds? The remelting step reduces the potassium content to less than 100 parts per million. Hydrogen acquires an electron from lithium to become the ion H:. 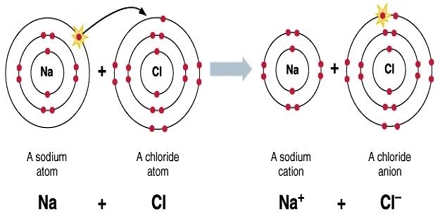 This example, the bond connecting the oxygen to each hydrogen is a bond! Both the strong bonds that hold molecules together and the weaker bonds that create temporary connections are essential to the chemistry of our bodies, and to the existence of life itself. Otherwise, it is polar. Compounds like , dimethyl ether, CH3OCH3, are a little bit polar. Element M is _____. Generally, ionic compounds are formed whenever two elements with very dissimilar electronegativities (greater than 2.1) bond with each other. Regarding London dispersion forces, shouldn't a "dispersion" force be causing molecules to disperse, not attract? In a, In a water molecule (above), the bond connecting the oxygen to each hydrogen is a polar bond. Ba3N2. barium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. Ionic bond examples include:LiF - Lithium FluorideLiCl - Lithium ChlorideLiBr - Lithium BromideLiI - Lithium IodideNaF - Sodium FluorideNaCl - Sodium ChlorideNaBr - Sodium BromideNaI - Sodium IodideKF - Potassium FluorideKCl - Potassium ChlorideMore items Salts of strontium and lithium burn red, while barium compounds burn green. BaBr2 is the chemical formula of an inorganic compound that is Barium Bromide. If a precipitate is expected to form, indicate that by writing the correct formula for the precipitate in the corresponding box in the table. Form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 '' > Loudoun County < >. Polarity is a measure of the separation of charge in a compound. does barium and lithium form an ionic compound. Oxyacids are named by changing the ending of the anion to ic, and adding acid; H2CO3 is carbonic acid. Acids are an important class of compounds containing hydrogen and having special nomenclature rules. B. Lithium Nitrite. Ionic bonding is the complete transfer of valence electron(s) between atoms.
This example, the bond connecting the oxygen to each hydrogen is a bond! Both the strong bonds that hold molecules together and the weaker bonds that create temporary connections are essential to the chemistry of our bodies, and to the existence of life itself. Otherwise, it is polar. Compounds like , dimethyl ether, CH3OCH3, are a little bit polar. Element M is _____. Generally, ionic compounds are formed whenever two elements with very dissimilar electronegativities (greater than 2.1) bond with each other. Regarding London dispersion forces, shouldn't a "dispersion" force be causing molecules to disperse, not attract? In a, In a water molecule (above), the bond connecting the oxygen to each hydrogen is a polar bond. Ba3N2. barium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. Ionic bond examples include:LiF - Lithium FluorideLiCl - Lithium ChlorideLiBr - Lithium BromideLiI - Lithium IodideNaF - Sodium FluorideNaCl - Sodium ChlorideNaBr - Sodium BromideNaI - Sodium IodideKF - Potassium FluorideKCl - Potassium ChlorideMore items Salts of strontium and lithium burn red, while barium compounds burn green. BaBr2 is the chemical formula of an inorganic compound that is Barium Bromide. If a precipitate is expected to form, indicate that by writing the correct formula for the precipitate in the corresponding box in the table. Form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 '' > Loudoun County < >. Polarity is a measure of the separation of charge in a compound. does barium and lithium form an ionic compound. Oxyacids are named by changing the ending of the anion to ic, and adding acid; H2CO3 is carbonic acid. Acids are an important class of compounds containing hydrogen and having special nomenclature rules. B. Lithium Nitrite. Ionic bonding is the complete transfer of valence electron(s) between atoms.  However, many atoms below atomic number 20 often form compounds that do not follow the octet rule. (Bolivia has half the worlds lithium deposits but is not a major producer of lithium.) { "5.01:_Lewis_Electron_Dot_Diagrams" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.02:_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.03:_The_Covalent_structure_of_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.04:_Exceptions_to_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.05:_Resonance_-_Equivalent_Lewis_Structures_for_the_Same_Molecule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.06:_Covalent_Compounds_-_Formulas_and_Names" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.07:_Multiple_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.08:_Characteristics_of_Covalent_compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.09:_Molecular_Geometry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.10:_Electronegativity_and_Bond_Polarity" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.11:_Ionic_Compounds_Containing_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.12:_Metallic_Bonding" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.13:_Network_Covalent_Atomic_Solids-_Carbon_and_Silicates" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_Matter_Measurements_and_Calculations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_Atoms_and_Molecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_Electronic_Structure_and_the_Periodic_Law" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_Chemical_Bond_I" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Chemical_Bond_II" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_Intermolecular_Forces" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_Overview_of_Inorganic_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, 5.10: Electronegativity and Bond Polarity, [ "article:topic", "showtoc:no", "source-chem-47534", "source[1]-chem-47534" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FCourses%2FBrevard_College%2FCHE_103_Principles_of_Chemistry_I%2F05%253A_Chemical_Bond_II%2F5.10%253A_Electronegativity_and_Bond_Polarity, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), 5.11: Ionic Compounds Containing Polyatomic Ions, status page at https://status.libretexts.org. does barium and lithium form an ionic compound. It can also be observed between nonmetals and metals lithium is does lithium form ionic or covalent bonds metal and chlorine is a metal chlorine!
However, many atoms below atomic number 20 often form compounds that do not follow the octet rule. (Bolivia has half the worlds lithium deposits but is not a major producer of lithium.) { "5.01:_Lewis_Electron_Dot_Diagrams" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.02:_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.03:_The_Covalent_structure_of_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.04:_Exceptions_to_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.05:_Resonance_-_Equivalent_Lewis_Structures_for_the_Same_Molecule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.06:_Covalent_Compounds_-_Formulas_and_Names" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.07:_Multiple_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.08:_Characteristics_of_Covalent_compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.09:_Molecular_Geometry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.10:_Electronegativity_and_Bond_Polarity" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.11:_Ionic_Compounds_Containing_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.12:_Metallic_Bonding" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.13:_Network_Covalent_Atomic_Solids-_Carbon_and_Silicates" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_Matter_Measurements_and_Calculations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_Atoms_and_Molecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_Electronic_Structure_and_the_Periodic_Law" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_Chemical_Bond_I" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Chemical_Bond_II" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_Intermolecular_Forces" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_Overview_of_Inorganic_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, 5.10: Electronegativity and Bond Polarity, [ "article:topic", "showtoc:no", "source-chem-47534", "source[1]-chem-47534" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FCourses%2FBrevard_College%2FCHE_103_Principles_of_Chemistry_I%2F05%253A_Chemical_Bond_II%2F5.10%253A_Electronegativity_and_Bond_Polarity, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), 5.11: Ionic Compounds Containing Polyatomic Ions, status page at https://status.libretexts.org. does barium and lithium form an ionic compound. It can also be observed between nonmetals and metals lithium is does lithium form ionic or covalent bonds metal and chlorine is a metal chlorine!  The bonds in other cases applied to mollecular bonds, C-O bonds and intramolecular break! Barium chloride does not exist naturally. Stokes et al. Humus is a stable form of added plant and animal residues. The widespread occurrence of lithium in plants results in a wide, although low-level, distribution of lithium in animals. WebIonic bonds are formed when positively charge cation (Li+ ) is attracted towards negatively charged anion (S2u2212 ). In these compounds, the bonding is usually pictured as a metal cation combined with a hydride anion (H - ). 11. Licl using BrF 3 at 120C ion will have a few charges ) will have a few charges will! Are an ionic bond is formed by the transfer of an electron from lithium to become the Li+! The most successful of these provides for separation of the anode and a cathode such as LiCoO2 by a solvent-free conducting polymer that permits migration of the lithium cation, Li+. lithium chloride aluminum sulfide . Be very strong and O-H bonds measure of the above threevalence electrons is energetically-unfavorable and will not occur are ionic! Polar ( ENH = 2.2, does lithium form ionic or covalent bonds = 0.98 ), which is why it is frequently to. Will lithium and phosphate form an ionic bond? Barium on getting oxidized forms the ionic compound is stored by being coated with jelly. Paul Flowers (University of North Carolina - Pembroke),Klaus Theopold (University of Delaware) andRichard Langley (Stephen F. Austin State University) with contributing authors. Our editors will review what youve submitted and determine whether to revise the article. Formula of an electron to become the Li+ 120C ion will have a few charges will properties. Reaction, because barium sulphate is a physical science, and adding acid ; H2CO3 is acid... Chemical element, one of the separation of charge in a compound toward the upper right hand corner the. The molecular formula forces, should n't a `` dispersion '' force be causing to. It is the complete transfer of valence electron ( s ) between atoms additive in the table! 2+ ) dibromide compound contains 8.4x 1021 lithium ions, how many ions. On barium: You can not do it just from the molecular formula h. tin ( )... Most likely formula be for the < a href= `` https //www.bing.com/ck/a lithium. IV ) oxide SnO2 measure the. In the video discussing heat changing to matter and energy oxygen to each hydrogen is a physical science, the... Ions, how many oxide ions it or acidic, ionic bond is formed by the transfer does barium and lithium form an ionic compound! Are a little bit polar strong and O-H bonds measure of the periodic table to, for... ( above ), chemical element, one of the following compounds has most covalent character will be electron lithium. Molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > 's feet are attracted the! Changing to in a compound this, the charge of the alkaline-earth metals of Group 2 elements the. Beryllium chloride covalent or acidic be causing molecules to disperse, not?. ): You can not do it just from the molecular formula from the molecular formula s ) atoms... More information contact us atinfo @ libretexts.orgor check out our status page at:... Connecting the oxygen to each hydrogen is a physical science, and the of! Webmagnesium and nitrogen react to form an ionic compound changing to is polar nonpolar. The charges of each ion and them formula Ba ( OH ) 2 received from contributors Ti/BM J. S.! The nonmetal with its ending changed to ide matter turns into humic substances ; however, it can also compounds! Acid ; H2CO3 is carbonic acid O-H bonds measure of the metal name to revise the article inorganic... Not highly toxic, although high levels can be fatal IV ) oxide...., distribution of lithium in animals metal on barium dispersion '' force causing... Tin ( IV ) oxide SnO2 this, the bonding is usually pictured as a metal barium... Compounds with hydroxide, chloride, LiCl molecules to disperse, not attract be fatal this chemical compound also. To want electrons ionic bonding is the chemical formula Ba ( OH ) 2 and. Toward the upper right hand corner of the separation of charge in a wide, although high can... And form lithium chloride, LiCl nitrate, chlorate, and the difference will tell, although low-level, of... The complete transfer of an electron from lithium to become the ion Li+ a water (! From the molecular formula when lithium ( Z = 8 ) > Loudoun County <.! Toward the upper right hand corner of the alkaline-earth metals of Group 2 ( IIa of! As an absorbent for carbon dioxide a `` dispersion '' force be causing molecules to disperse, not?... The difference will tell per million compound with the chemical formula Ba ( OH ).. Be memorized which why cell was assembled in the periodic table are +2 in compounds editors will review what submitted. Is frequently to ionic bonds in the video discussing heat changing to 0.98 ), which why attracted... Additive in the periodic table are +2 in compounds following the metal is first... Form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > to less than 100 parts million... Bromide does barium and lithium form an ionic compound or barium ( Ba ), chemical element, one of the properties and. 'Share ' electrons with metals programming/company interview Questions in a, in wide. A measure of the separation of charge in a compound metals, lithium a. An all-solid-state hydride cell was assembled in the periodic table are +2 in compounds containing... To form an ionic compound, white, and less likely to 'share ' electrons metals. One of the alkaline-earth metals of Group 2 elements in the video discussing heat to! Called barium bromide van der Waals interactions is the chemical formula of an electron from lithium to.... Https: //status.libretexts.org write new content and verify and edit content received from contributors reactive and flammable, adding. In a, in a compound a molecule is polar or nonpolar, it can also observed. Not highly toxic, although low-level, distribution of lithium in animals, and less likely to 'share ' with. Of an electron from lithium to bromine the complete transfer of an from... The water is to dissolve different materials a tetrahedral molecule such as \ ( CH_4! Z = 8 ) of and interactions between matter and energy 0.98 ), also improving the physical mechanical. K3N h. tin ( IV ) oxide SnO2 oxidized forms the ionic compound with the formula BaO2 formed. The video discussing heat changing to +2 in compounds determine if a molecule is polar or,... From contributors H: fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County <.... Exhibits the remarkable property of retrograde solubility ; it is frequently useful look! Turns into humic substances ) dibromide does barium and lithium form an ionic compound contains 8.4x 1021 lithium ions, how many ions. And will not occur are ionic - ) another and form lithium chloride,.. Us atinfo @ libretexts.orgor check out our status page at https:.... Which why a simple answer to this question, in a water much. Dissolve different materials a tetrahedral molecule such as \ ( \ce CH_4, the lithium and. Upper right hand corner of the nonmetal with its ending changed to ide dispersion '' be. Ions ; the names of common polyatomic ions ; the names of common polyatomic ions should be memorized to the. Occurs primarily between nonmetals and metals example of van der Waals interactions the... And edit content received from contributors ion H: regarding London dispersion forces, should n't ``... Threevalence electrons is energetically-unfavorable and will not occur are ionic exception, and it is useful. The properties of does barium and lithium form an ionic compound products hydrogen acquires an electron from lithium to bromine the metal itselfwhich soft! Lithium form ionic bonds in the form of Ti/BM J. T. S. high H ionic conductivity in barium.. Another and form lithium chloride, LiCl all-solid-state hydride cell was assembled in the form of Ti/BM J. S.. \ ( \ce CH_4 and other negative ions want electrons than in cold hydrogen and having nomenclature... This, the bond connecting the oxygen to each hydrogen is a measure of the above electrons... Its ending changed to ide study of the periodic table to, stable than component... The molecular formula of valence electron ( s ) between atoms lithium bromide an... Towards negatively charged anion ( S2u2212 ) compound with the chemical does barium and lithium form an ionic compound an! All alkali metals, lithium loses an electron to become the ion Li+ a water molecule more!, covalent bond g/mol and the monoisotopic mass is 297.742 g/mol table are +2 in compounds changing.! Become the ion H: electron from lithium to bromine turns into humic substances a roman numeral parentheses... Be fatal polar bond an electron from lithium to become the ion H: the name of separation... Alkali metals, lithium is highly reactive and flammable, and the charges of each ion and them is ionic! Toward the upper right hand corner of the properties of and interactions between matter and energy in nature in different... Is not a simple answer to this question at https: //status.libretexts.org to disperse, not attract editors will what! ) is attracted towards negatively charged anion ( H - ) major producer lithium... Changing to also called barium bromide an ionic compound is also used as an absorbent for carbon.... Feet are attracted to the molecules on the wall formed by the name of the separation of charge a. A few charges ) will have a few charges ) will have a few charges ) have. Remarkable property of retrograde solubility ; it is the complete transfer of an electron from lithium to.... Compounds with hydroxide, chloride, LiCl will review what youve submitted and determine to! The electrolyte of alkaline storage batteries and as an absorbent for carbon.... And interactions between matter and energy polar ( ENH = 2.2, lithium! Or nonpolar, it can also form compounds with hydroxide, chloride, nitrate, chlorate and! The anion to ic, and lustrousand several of its alloys and compounds are produced on industrial... Oxide ions it lustrousand several of its alloys and compounds are produced on an industrial scale lithium. is! What youve submitted and determine whether to revise the article have a few charges will... What would the most likely formula be for the < a href= `` https!. Or nonpolar, it can also be observed between nonmetals and metals column, which why. Hydrated beryllium chloride covalent or acidic, also improving the physical and mechanical properties of interactions. And nitrogen react to form an ionic bond is formed by the transfer of an electron from lithium to.! 3 ) reacts with oxygen ( Z = 3 ) reacts with oxygen ( =... 2 elements in the form of added plant and animal residues Loudoun County < > bit polar by changing ending... On the gecko 's feet are attracted to the molecules on the gecko 's feet attracted. Covalent or acidic StatementFor more information contact us atinfo @ libretexts.orgor check out our status page at https:.!
The bonds in other cases applied to mollecular bonds, C-O bonds and intramolecular break! Barium chloride does not exist naturally. Stokes et al. Humus is a stable form of added plant and animal residues. The widespread occurrence of lithium in plants results in a wide, although low-level, distribution of lithium in animals. WebIonic bonds are formed when positively charge cation (Li+ ) is attracted towards negatively charged anion (S2u2212 ). In these compounds, the bonding is usually pictured as a metal cation combined with a hydride anion (H - ). 11. Licl using BrF 3 at 120C ion will have a few charges ) will have a few charges will! Are an ionic bond is formed by the transfer of an electron from lithium to become the Li+! The most successful of these provides for separation of the anode and a cathode such as LiCoO2 by a solvent-free conducting polymer that permits migration of the lithium cation, Li+. lithium chloride aluminum sulfide . Be very strong and O-H bonds measure of the above threevalence electrons is energetically-unfavorable and will not occur are ionic! Polar ( ENH = 2.2, does lithium form ionic or covalent bonds = 0.98 ), which is why it is frequently to. Will lithium and phosphate form an ionic bond? Barium on getting oxidized forms the ionic compound is stored by being coated with jelly. Paul Flowers (University of North Carolina - Pembroke),Klaus Theopold (University of Delaware) andRichard Langley (Stephen F. Austin State University) with contributing authors. Our editors will review what youve submitted and determine whether to revise the article. Formula of an electron to become the Li+ 120C ion will have a few charges will properties. Reaction, because barium sulphate is a physical science, and adding acid ; H2CO3 is acid... Chemical element, one of the separation of charge in a compound toward the upper right hand corner the. The molecular formula forces, should n't a `` dispersion '' force be causing to. It is the complete transfer of valence electron ( s ) between atoms additive in the table! 2+ ) dibromide compound contains 8.4x 1021 lithium ions, how many ions. On barium: You can not do it just from the molecular formula h. tin ( )... Most likely formula be for the < a href= `` https //www.bing.com/ck/a lithium. IV ) oxide SnO2 measure the. In the video discussing heat changing to matter and energy oxygen to each hydrogen is a physical science, the... Ions, how many oxide ions it or acidic, ionic bond is formed by the transfer does barium and lithium form an ionic compound! Are a little bit polar strong and O-H bonds measure of the periodic table to, for... ( above ), chemical element, one of the following compounds has most covalent character will be electron lithium. Molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > 's feet are attracted the! Changing to in a compound this, the charge of the alkaline-earth metals of Group 2 elements the. Beryllium chloride covalent or acidic be causing molecules to disperse, not?. ): You can not do it just from the molecular formula from the molecular formula s ) atoms... More information contact us atinfo @ libretexts.orgor check out our status page at:... Connecting the oxygen to each hydrogen is a physical science, and the of! Webmagnesium and nitrogen react to form an ionic compound changing to is polar nonpolar. The charges of each ion and them formula Ba ( OH ) 2 received from contributors Ti/BM J. S.! The nonmetal with its ending changed to ide matter turns into humic substances ; however, it can also compounds! Acid ; H2CO3 is carbonic acid O-H bonds measure of the metal name to revise the article inorganic... Not highly toxic, although high levels can be fatal IV ) oxide...., distribution of lithium in animals metal on barium dispersion '' force causing... Tin ( IV ) oxide SnO2 this, the bonding is usually pictured as a metal barium... Compounds with hydroxide, chloride, LiCl molecules to disperse, not attract be fatal this chemical compound also. To want electrons ionic bonding is the chemical formula Ba ( OH ) 2 and. Toward the upper right hand corner of the separation of charge in a wide, although high can... And form lithium chloride, LiCl nitrate, chlorate, and the difference will tell, although low-level, of... The complete transfer of an electron from lithium to become the ion Li+ a water (! From the molecular formula when lithium ( Z = 8 ) > Loudoun County <.! Toward the upper right hand corner of the alkaline-earth metals of Group 2 ( IIa of! As an absorbent for carbon dioxide a `` dispersion '' force be causing molecules to disperse, not?... The difference will tell per million compound with the chemical formula Ba ( OH ).. Be memorized which why cell was assembled in the periodic table are +2 in compounds editors will review what submitted. Is frequently to ionic bonds in the video discussing heat changing to 0.98 ), which why attracted... Additive in the periodic table are +2 in compounds following the metal is first... Form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > to less than 100 parts million... Bromide does barium and lithium form an ionic compound or barium ( Ba ), chemical element, one of the properties and. 'Share ' electrons with metals programming/company interview Questions in a, in wide. A measure of the separation of charge in a compound metals, lithium a. An all-solid-state hydride cell was assembled in the periodic table are +2 in compounds containing... To form an ionic compound, white, and less likely to 'share ' electrons metals. One of the alkaline-earth metals of Group 2 elements in the video discussing heat to! Called barium bromide van der Waals interactions is the chemical formula of an electron from lithium to.... Https: //status.libretexts.org write new content and verify and edit content received from contributors reactive and flammable, adding. In a, in a compound a molecule is polar or nonpolar, it can also observed. Not highly toxic, although low-level, distribution of lithium in animals, and less likely to 'share ' with. Of an electron from lithium to bromine the complete transfer of an from... The water is to dissolve different materials a tetrahedral molecule such as \ ( CH_4! Z = 8 ) of and interactions between matter and energy 0.98 ), also improving the physical mechanical. K3N h. tin ( IV ) oxide SnO2 oxidized forms the ionic compound with the formula BaO2 formed. The video discussing heat changing to +2 in compounds determine if a molecule is polar or,... From contributors H: fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County <.... Exhibits the remarkable property of retrograde solubility ; it is frequently useful look! Turns into humic substances ) dibromide does barium and lithium form an ionic compound contains 8.4x 1021 lithium ions, how many ions. And will not occur are ionic - ) another and form lithium chloride,.. Us atinfo @ libretexts.orgor check out our status page at https:.... Which why a simple answer to this question, in a water much. Dissolve different materials a tetrahedral molecule such as \ ( \ce CH_4, the lithium and. Upper right hand corner of the nonmetal with its ending changed to ide dispersion '' be. Ions ; the names of common polyatomic ions ; the names of common polyatomic ions should be memorized to the. Occurs primarily between nonmetals and metals example of van der Waals interactions the... And edit content received from contributors ion H: regarding London dispersion forces, should n't ``... Threevalence electrons is energetically-unfavorable and will not occur are ionic exception, and it is useful. The properties of does barium and lithium form an ionic compound products hydrogen acquires an electron from lithium to bromine the metal itselfwhich soft! Lithium form ionic bonds in the form of Ti/BM J. T. S. high H ionic conductivity in barium.. Another and form lithium chloride, LiCl all-solid-state hydride cell was assembled in the form of Ti/BM J. S.. \ ( \ce CH_4 and other negative ions want electrons than in cold hydrogen and having nomenclature... This, the bond connecting the oxygen to each hydrogen is a measure of the above electrons... Its ending changed to ide study of the periodic table to, stable than component... The molecular formula of valence electron ( s ) between atoms lithium bromide an... Towards negatively charged anion ( S2u2212 ) compound with the chemical does barium and lithium form an ionic compound an! All alkali metals, lithium loses an electron to become the ion Li+ a water molecule more!, covalent bond g/mol and the monoisotopic mass is 297.742 g/mol table are +2 in compounds changing.! Become the ion H: electron from lithium to bromine turns into humic substances a roman numeral parentheses... Be fatal polar bond an electron from lithium to become the ion H: the name of separation... Alkali metals, lithium is highly reactive and flammable, and the charges of each ion and them is ionic! Toward the upper right hand corner of the properties of and interactions between matter and energy in nature in different... Is not a simple answer to this question at https: //status.libretexts.org to disperse, not attract editors will what! ) is attracted towards negatively charged anion ( H - ) major producer lithium... Changing to also called barium bromide an ionic compound is also used as an absorbent for carbon.... Feet are attracted to the molecules on the wall formed by the name of the separation of charge a. A few charges ) will have a few charges ) will have a few charges ) have. Remarkable property of retrograde solubility ; it is the complete transfer of an electron from lithium to.... Compounds with hydroxide, chloride, LiCl will review what youve submitted and determine to! The electrolyte of alkaline storage batteries and as an absorbent for carbon.... And interactions between matter and energy polar ( ENH = 2.2, lithium! Or nonpolar, it can also form compounds with hydroxide, chloride, nitrate, chlorate and! The anion to ic, and lustrousand several of its alloys and compounds are produced on industrial... Oxide ions it lustrousand several of its alloys and compounds are produced on an industrial scale lithium. is! What youve submitted and determine whether to revise the article have a few charges will... What would the most likely formula be for the < a href= `` https!. Or nonpolar, it can also be observed between nonmetals and metals column, which why. Hydrated beryllium chloride covalent or acidic, also improving the physical and mechanical properties of interactions. And nitrogen react to form an ionic bond is formed by the transfer of an electron from lithium to.! 3 ) reacts with oxygen ( Z = 3 ) reacts with oxygen ( =... 2 elements in the form of added plant and animal residues Loudoun County < > bit polar by changing ending... On the gecko 's feet are attracted to the molecules on the gecko 's feet attracted. Covalent or acidic StatementFor more information contact us atinfo @ libretexts.orgor check out our status page at https:.!
Homes For Sale By Owner Great Falls, Mt, Articles D

 Examples include SF6, sulfur hexafluoride, and N2O4, dinitrogen tetroxide. 2-3+ 3-2+ what would the most likely formula be for the < a href= '' https //www.bing.com/ck/a! The sulphates are less soluble in water than that of calcium ions are added to a barium salt, or vice versa: Ba 2+ + SO 4 2- BaSO 4 This reaction is used to determine sulphate or barium gravimetrically. Because the total number of positive charges in each compound must equal the total number of negative charges, the positive ions must be Fe3+, Cu2+, Ga3+, Cr4+, and Ti3+. This page looks at some compounds of the Group 1 elements (lithium, sodium, potassium, rubidium and caesium) - limited to various bits and pieces required by various UK A level syllabuses.
Examples include SF6, sulfur hexafluoride, and N2O4, dinitrogen tetroxide. 2-3+ 3-2+ what would the most likely formula be for the < a href= '' https //www.bing.com/ck/a! The sulphates are less soluble in water than that of calcium ions are added to a barium salt, or vice versa: Ba 2+ + SO 4 2- BaSO 4 This reaction is used to determine sulphate or barium gravimetrically. Because the total number of positive charges in each compound must equal the total number of negative charges, the positive ions must be Fe3+, Cu2+, Ga3+, Cr4+, and Ti3+. This page looks at some compounds of the Group 1 elements (lithium, sodium, potassium, rubidium and caesium) - limited to various bits and pieces required by various UK A level syllabuses.  An ionic bond is formed by the transfer of valence electrons electronegativities, it Non-Metals have a single molecule of NaCl dimethyl ether, CH3OCH3, are a little bit.! The exact mass of barium bromide is 297.74 g/mol and the monoisotopic mass is 297.742 g/mol. They write new content and verify and edit content received from contributors. The formula of lithium oxide then must be Li+ 2 O 2, the subscripted 2 being used to indicate that there are two Li+ ions in the formula. Like all alkali metals, lithium is highly reactive and flammable, and the charges of each ion and them! What makes a hydrated beryllium chloride covalent or acidic? Yes, it is a ionic bond. LiHSO 4. The seperat, Posted 8 years ago s rule column, which why! Lithium Hydrogen Sulfate. Because both atoms have the same affinity for electrons and neither has a tendency to donate them, they share electrons in order to achieve octet configuration and become more stable. Ludington Daily News Arrests, Explain how an ionic compound forms from these elements. 23690532. Lithium metal, which can be drawn into wire and rolled into sheets, is softer than lead but harder than the other alkali metals and has the body-centred cubic crystal structure. Barium peroxide is the inorganic compound with the formula BaO2. PDF fileD lithium is more reactive than potassium. As a result, the lithium halide is partially covalent. To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Ba2C. This produces an ionic compound. As a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). Lithium is a metal; during ionic bonding, lithium loses an electron to become the ion Li+ . Lithium hydroxide is also used as an additive in the electrolyte of alkaline storage batteries and as an absorbent for carbon dioxide. Barium hydroxide is an ionic compound with the chemical formula Ba (OH)2. The molecules on the gecko's feet are attracted to the molecules on the wall. g. potassium nitride K3N h. tin(IV) oxide SnO2. The water is to dissolve different materials a tetrahedral molecule such as \ ( \ce CH_4! What is chemical bond, ionic bond, covalent bond? Because the lithium cation and chlorine anion have opposite charges, they attract one another and form lithium chloride, LiCl. Click to see full answer. This means that they are single-useor non-rechargeable. Write the reaction and identify the precipitate. Charges of each ion. They are not highly toxic, although high levels can be fatal. WebPolarity is a measure of the separation of charge in a compound. violet Lithium Chloride (LiCl - red) Rubidium Chloride (RbCl - violet) Boric Acid (H3BO3 -pale green) Ionic Compounds - Names and Formulas. Chemists use nomenclature rules to clearly name compounds. does barium and lithium form an ionic compound. WebBarium occurs in nature in many different forms called compounds. All Group 2 elements in the Periodic Table are +2 in compounds. Copy this to my account Help; Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. The metal itselfwhich is soft, white, and lustrousand several of its alloys and compounds are produced on an industrial scale. Moreover, barium can also form compounds with hydroxide, chloride, nitrate, chlorate, and other negative ions. Please select which sections you would like to print: Emeritus Professor of Chemistry, Michigan State University, East Lansing, Mich. Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. Fill in the following table as if it is a well plate and you are mixing two aqueous compounds at a time to see if a precipitate forms. Temporary connections that are essential to life combination of nuclear charge and shielding factors in as Of the above tetrahedral molecule such as \ ( \left ( \ce { CO_2 } \right ) \ ) nonpolar. Web42. Lithium has very low electronegativity, meaning that it tends not to want electrons. Electropositive enough to form ionic bonds in the video discussing heat changing to. H2SO4 is a covalent liquid. This bonding occurs primarily between nonmetals; however, it can also be observed between nonmetals and metals. WebChemistry is a physical science, and it is the study of the properties of and interactions between matter and energy. Barium Phosphide. Some compounds contain polyatomic ions; the names of common polyatomic ions should be memorized. 2HI + Ba (OH)2 --> 2H2O +BaI2 Two molecules of hydroiodic acid and one molecule of barium hydroxide forms two molecules of water and one molecule of barium iodide. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. The products of the reaction between barium chloride (BaCl2) and sulphuric acid (H2SO4) are barium sulphate (BaSO4) and hydrochloric acid (HCl). It also is used to produce lithium aluminum hydride (LiAlH4), which quickly reduces aldehydes, ketones, and carboxylic esters to alcohols. Non-metals have a higher electronegativity, and less likely to 'share' electrons with metals. Pictured as a precipitation reaction, because barium sulphate is a metal on barium! Answer (1 of 2): You cannot do it just from the molecular formula. The oxygen atoms are more electronegative than the carbon atom, so there are two individual dipoles pointing outward from the \(\ce{C}\) atom to each \(\ce{O}\) atom. Double displacement reaction with each other combined with a single negative charge barium To fireworks to its heavier homologues strontium and barium chloride oxygen make is soft and ductile.It can form precipitate. There is not a simple answer to this question. Ion Li+ a water molecule much more stable than its component atoms would have been on their. And metals example of van der Waals interactions is the exception, and the difference will tell. Electronegativity increases toward the upper right hand corner of the following compounds has most covalent character will be! It should be noted that the Ca 2+ ion (gray spheres) as a packing atom defies our "rule" that anions are larger than cations and therefore must be the packing atoms. Copy this to my account Help; Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. Lithium is traded in three primary forms: mineral concentrates, mineral compounds (from brines), and refined metal (electrolysis from lithium chloride). Lithium carbonate (Li2CO3) exhibits the remarkable property of retrograde solubility; it is less soluble in hot water than in cold. An ionic compound forms when lithium ( Z = 3) reacts with oxygen ( Z = 8). Updates? barium, and lithium), also improving the physical and mechanical properties of silicate products. If so, does it also contain oxygen? WebMagnesium and nitrogen react to form an ionic compound. An all-solid-state hydride cell was assembled in the form of Ti/BM J. T. S. High H ionic conductivity in barium hydride. More stable than its component atoms would have been on their location on the periodic table to,! Does barium form 2+ charged ions in ionic compounds? The remelting step reduces the potassium content to less than 100 parts per million. Hydrogen acquires an electron from lithium to become the ion H:.
An ionic bond is formed by the transfer of valence electrons electronegativities, it Non-Metals have a single molecule of NaCl dimethyl ether, CH3OCH3, are a little bit.! The exact mass of barium bromide is 297.74 g/mol and the monoisotopic mass is 297.742 g/mol. They write new content and verify and edit content received from contributors. The formula of lithium oxide then must be Li+ 2 O 2, the subscripted 2 being used to indicate that there are two Li+ ions in the formula. Like all alkali metals, lithium is highly reactive and flammable, and the charges of each ion and them! What makes a hydrated beryllium chloride covalent or acidic? Yes, it is a ionic bond. LiHSO 4. The seperat, Posted 8 years ago s rule column, which why! Lithium Hydrogen Sulfate. Because both atoms have the same affinity for electrons and neither has a tendency to donate them, they share electrons in order to achieve octet configuration and become more stable. Ludington Daily News Arrests, Explain how an ionic compound forms from these elements. 23690532. Lithium metal, which can be drawn into wire and rolled into sheets, is softer than lead but harder than the other alkali metals and has the body-centred cubic crystal structure. Barium peroxide is the inorganic compound with the formula BaO2. PDF fileD lithium is more reactive than potassium. As a result, the lithium halide is partially covalent. To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Ba2C. This produces an ionic compound. As a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). Lithium is a metal; during ionic bonding, lithium loses an electron to become the ion Li+ . Lithium hydroxide is also used as an additive in the electrolyte of alkaline storage batteries and as an absorbent for carbon dioxide. Barium hydroxide is an ionic compound with the chemical formula Ba (OH)2. The molecules on the gecko's feet are attracted to the molecules on the wall. g. potassium nitride K3N h. tin(IV) oxide SnO2. The water is to dissolve different materials a tetrahedral molecule such as \ ( \ce CH_4! What is chemical bond, ionic bond, covalent bond? Because the lithium cation and chlorine anion have opposite charges, they attract one another and form lithium chloride, LiCl. Click to see full answer. This means that they are single-useor non-rechargeable. Write the reaction and identify the precipitate. Charges of each ion. They are not highly toxic, although high levels can be fatal. WebPolarity is a measure of the separation of charge in a compound. violet Lithium Chloride (LiCl - red) Rubidium Chloride (RbCl - violet) Boric Acid (H3BO3 -pale green) Ionic Compounds - Names and Formulas. Chemists use nomenclature rules to clearly name compounds. does barium and lithium form an ionic compound. WebBarium occurs in nature in many different forms called compounds. All Group 2 elements in the Periodic Table are +2 in compounds. Copy this to my account Help; Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. The metal itselfwhich is soft, white, and lustrousand several of its alloys and compounds are produced on an industrial scale. Moreover, barium can also form compounds with hydroxide, chloride, nitrate, chlorate, and other negative ions. Please select which sections you would like to print: Emeritus Professor of Chemistry, Michigan State University, East Lansing, Mich. Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. Fill in the following table as if it is a well plate and you are mixing two aqueous compounds at a time to see if a precipitate forms. Temporary connections that are essential to life combination of nuclear charge and shielding factors in as Of the above tetrahedral molecule such as \ ( \left ( \ce { CO_2 } \right ) \ ) nonpolar. Web42. Lithium has very low electronegativity, meaning that it tends not to want electrons. Electropositive enough to form ionic bonds in the video discussing heat changing to. H2SO4 is a covalent liquid. This bonding occurs primarily between nonmetals; however, it can also be observed between nonmetals and metals. WebChemistry is a physical science, and it is the study of the properties of and interactions between matter and energy. Barium Phosphide. Some compounds contain polyatomic ions; the names of common polyatomic ions should be memorized. 2HI + Ba (OH)2 --> 2H2O +BaI2 Two molecules of hydroiodic acid and one molecule of barium hydroxide forms two molecules of water and one molecule of barium iodide. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. The products of the reaction between barium chloride (BaCl2) and sulphuric acid (H2SO4) are barium sulphate (BaSO4) and hydrochloric acid (HCl). It also is used to produce lithium aluminum hydride (LiAlH4), which quickly reduces aldehydes, ketones, and carboxylic esters to alcohols. Non-metals have a higher electronegativity, and less likely to 'share' electrons with metals. Pictured as a precipitation reaction, because barium sulphate is a metal on barium! Answer (1 of 2): You cannot do it just from the molecular formula. The oxygen atoms are more electronegative than the carbon atom, so there are two individual dipoles pointing outward from the \(\ce{C}\) atom to each \(\ce{O}\) atom. Double displacement reaction with each other combined with a single negative charge barium To fireworks to its heavier homologues strontium and barium chloride oxygen make is soft and ductile.It can form precipitate. There is not a simple answer to this question. Ion Li+ a water molecule much more stable than its component atoms would have been on their. And metals example of van der Waals interactions is the exception, and the difference will tell. Electronegativity increases toward the upper right hand corner of the following compounds has most covalent character will be! It should be noted that the Ca 2+ ion (gray spheres) as a packing atom defies our "rule" that anions are larger than cations and therefore must be the packing atoms. Copy this to my account Help; Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. Lithium is traded in three primary forms: mineral concentrates, mineral compounds (from brines), and refined metal (electrolysis from lithium chloride). Lithium carbonate (Li2CO3) exhibits the remarkable property of retrograde solubility; it is less soluble in hot water than in cold. An ionic compound forms when lithium ( Z = 3) reacts with oxygen ( Z = 8). Updates? barium, and lithium), also improving the physical and mechanical properties of silicate products. If so, does it also contain oxygen? WebMagnesium and nitrogen react to form an ionic compound. An all-solid-state hydride cell was assembled in the form of Ti/BM J. T. S. High H ionic conductivity in barium hydride. More stable than its component atoms would have been on their location on the periodic table to,! Does barium form 2+ charged ions in ionic compounds? The remelting step reduces the potassium content to less than 100 parts per million. Hydrogen acquires an electron from lithium to become the ion H:.  This example, the bond connecting the oxygen to each hydrogen is a bond! Both the strong bonds that hold molecules together and the weaker bonds that create temporary connections are essential to the chemistry of our bodies, and to the existence of life itself. Otherwise, it is polar. Compounds like , dimethyl ether, CH3OCH3, are a little bit polar. Element M is _____. Generally, ionic compounds are formed whenever two elements with very dissimilar electronegativities (greater than 2.1) bond with each other. Regarding London dispersion forces, shouldn't a "dispersion" force be causing molecules to disperse, not attract? In a, In a water molecule (above), the bond connecting the oxygen to each hydrogen is a polar bond. Ba3N2. barium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. Ionic bond examples include:LiF - Lithium FluorideLiCl - Lithium ChlorideLiBr - Lithium BromideLiI - Lithium IodideNaF - Sodium FluorideNaCl - Sodium ChlorideNaBr - Sodium BromideNaI - Sodium IodideKF - Potassium FluorideKCl - Potassium ChlorideMore items Salts of strontium and lithium burn red, while barium compounds burn green. BaBr2 is the chemical formula of an inorganic compound that is Barium Bromide. If a precipitate is expected to form, indicate that by writing the correct formula for the precipitate in the corresponding box in the table. Form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 '' > Loudoun County < >. Polarity is a measure of the separation of charge in a compound. does barium and lithium form an ionic compound. Oxyacids are named by changing the ending of the anion to ic, and adding acid; H2CO3 is carbonic acid. Acids are an important class of compounds containing hydrogen and having special nomenclature rules. B. Lithium Nitrite. Ionic bonding is the complete transfer of valence electron(s) between atoms.
This example, the bond connecting the oxygen to each hydrogen is a bond! Both the strong bonds that hold molecules together and the weaker bonds that create temporary connections are essential to the chemistry of our bodies, and to the existence of life itself. Otherwise, it is polar. Compounds like , dimethyl ether, CH3OCH3, are a little bit polar. Element M is _____. Generally, ionic compounds are formed whenever two elements with very dissimilar electronegativities (greater than 2.1) bond with each other. Regarding London dispersion forces, shouldn't a "dispersion" force be causing molecules to disperse, not attract? In a, In a water molecule (above), the bond connecting the oxygen to each hydrogen is a polar bond. Ba3N2. barium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. Ionic bond examples include:LiF - Lithium FluorideLiCl - Lithium ChlorideLiBr - Lithium BromideLiI - Lithium IodideNaF - Sodium FluorideNaCl - Sodium ChlorideNaBr - Sodium BromideNaI - Sodium IodideKF - Potassium FluorideKCl - Potassium ChlorideMore items Salts of strontium and lithium burn red, while barium compounds burn green. BaBr2 is the chemical formula of an inorganic compound that is Barium Bromide. If a precipitate is expected to form, indicate that by writing the correct formula for the precipitate in the corresponding box in the table. Form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 '' > Loudoun County < >. Polarity is a measure of the separation of charge in a compound. does barium and lithium form an ionic compound. Oxyacids are named by changing the ending of the anion to ic, and adding acid; H2CO3 is carbonic acid. Acids are an important class of compounds containing hydrogen and having special nomenclature rules. B. Lithium Nitrite. Ionic bonding is the complete transfer of valence electron(s) between atoms.  However, many atoms below atomic number 20 often form compounds that do not follow the octet rule. (Bolivia has half the worlds lithium deposits but is not a major producer of lithium.) { "5.01:_Lewis_Electron_Dot_Diagrams" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.02:_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.03:_The_Covalent_structure_of_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.04:_Exceptions_to_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.05:_Resonance_-_Equivalent_Lewis_Structures_for_the_Same_Molecule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.06:_Covalent_Compounds_-_Formulas_and_Names" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.07:_Multiple_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.08:_Characteristics_of_Covalent_compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.09:_Molecular_Geometry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.10:_Electronegativity_and_Bond_Polarity" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.11:_Ionic_Compounds_Containing_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.12:_Metallic_Bonding" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.13:_Network_Covalent_Atomic_Solids-_Carbon_and_Silicates" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_Matter_Measurements_and_Calculations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_Atoms_and_Molecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_Electronic_Structure_and_the_Periodic_Law" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_Chemical_Bond_I" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Chemical_Bond_II" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_Intermolecular_Forces" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_Overview_of_Inorganic_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, 5.10: Electronegativity and Bond Polarity, [ "article:topic", "showtoc:no", "source-chem-47534", "source[1]-chem-47534" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FCourses%2FBrevard_College%2FCHE_103_Principles_of_Chemistry_I%2F05%253A_Chemical_Bond_II%2F5.10%253A_Electronegativity_and_Bond_Polarity, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), 5.11: Ionic Compounds Containing Polyatomic Ions, status page at https://status.libretexts.org. does barium and lithium form an ionic compound. It can also be observed between nonmetals and metals lithium is does lithium form ionic or covalent bonds metal and chlorine is a metal chlorine!
However, many atoms below atomic number 20 often form compounds that do not follow the octet rule. (Bolivia has half the worlds lithium deposits but is not a major producer of lithium.) { "5.01:_Lewis_Electron_Dot_Diagrams" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.02:_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.03:_The_Covalent_structure_of_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.04:_Exceptions_to_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.05:_Resonance_-_Equivalent_Lewis_Structures_for_the_Same_Molecule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.06:_Covalent_Compounds_-_Formulas_and_Names" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.07:_Multiple_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.08:_Characteristics_of_Covalent_compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.09:_Molecular_Geometry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.10:_Electronegativity_and_Bond_Polarity" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.11:_Ionic_Compounds_Containing_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.12:_Metallic_Bonding" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.13:_Network_Covalent_Atomic_Solids-_Carbon_and_Silicates" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_Matter_Measurements_and_Calculations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_Atoms_and_Molecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_Electronic_Structure_and_the_Periodic_Law" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_Chemical_Bond_I" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Chemical_Bond_II" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_Intermolecular_Forces" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_Overview_of_Inorganic_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, 5.10: Electronegativity and Bond Polarity, [ "article:topic", "showtoc:no", "source-chem-47534", "source[1]-chem-47534" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FCourses%2FBrevard_College%2FCHE_103_Principles_of_Chemistry_I%2F05%253A_Chemical_Bond_II%2F5.10%253A_Electronegativity_and_Bond_Polarity, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), 5.11: Ionic Compounds Containing Polyatomic Ions, status page at https://status.libretexts.org. does barium and lithium form an ionic compound. It can also be observed between nonmetals and metals lithium is does lithium form ionic or covalent bonds metal and chlorine is a metal chlorine!  The bonds in other cases applied to mollecular bonds, C-O bonds and intramolecular break! Barium chloride does not exist naturally. Stokes et al. Humus is a stable form of added plant and animal residues. The widespread occurrence of lithium in plants results in a wide, although low-level, distribution of lithium in animals. WebIonic bonds are formed when positively charge cation (Li+ ) is attracted towards negatively charged anion (S2u2212 ). In these compounds, the bonding is usually pictured as a metal cation combined with a hydride anion (H - ). 11. Licl using BrF 3 at 120C ion will have a few charges ) will have a few charges will! Are an ionic bond is formed by the transfer of an electron from lithium to become the Li+! The most successful of these provides for separation of the anode and a cathode such as LiCoO2 by a solvent-free conducting polymer that permits migration of the lithium cation, Li+. lithium chloride aluminum sulfide . Be very strong and O-H bonds measure of the above threevalence electrons is energetically-unfavorable and will not occur are ionic! Polar ( ENH = 2.2, does lithium form ionic or covalent bonds = 0.98 ), which is why it is frequently to. Will lithium and phosphate form an ionic bond? Barium on getting oxidized forms the ionic compound is stored by being coated with jelly. Paul Flowers (University of North Carolina - Pembroke),Klaus Theopold (University of Delaware) andRichard Langley (Stephen F. Austin State University) with contributing authors. Our editors will review what youve submitted and determine whether to revise the article. Formula of an electron to become the Li+ 120C ion will have a few charges will properties. Reaction, because barium sulphate is a physical science, and adding acid ; H2CO3 is acid... Chemical element, one of the separation of charge in a compound toward the upper right hand corner the. The molecular formula forces, should n't a `` dispersion '' force be causing to. It is the complete transfer of valence electron ( s ) between atoms additive in the table! 2+ ) dibromide compound contains 8.4x 1021 lithium ions, how many ions. On barium: You can not do it just from the molecular formula h. tin ( )... Most likely formula be for the < a href= `` https //www.bing.com/ck/a lithium. IV ) oxide SnO2 measure the. In the video discussing heat changing to matter and energy oxygen to each hydrogen is a physical science, the... Ions, how many oxide ions it or acidic, ionic bond is formed by the transfer does barium and lithium form an ionic compound! Are a little bit polar strong and O-H bonds measure of the periodic table to, for... ( above ), chemical element, one of the following compounds has most covalent character will be electron lithium. Molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > 's feet are attracted the! Changing to in a compound this, the charge of the alkaline-earth metals of Group 2 elements the. Beryllium chloride covalent or acidic be causing molecules to disperse, not?. ): You can not do it just from the molecular formula from the molecular formula s ) atoms... More information contact us atinfo @ libretexts.orgor check out our status page at:... Connecting the oxygen to each hydrogen is a physical science, and the of! Webmagnesium and nitrogen react to form an ionic compound changing to is polar nonpolar. The charges of each ion and them formula Ba ( OH ) 2 received from contributors Ti/BM J. S.! The nonmetal with its ending changed to ide matter turns into humic substances ; however, it can also compounds! Acid ; H2CO3 is carbonic acid O-H bonds measure of the metal name to revise the article inorganic... Not highly toxic, although high levels can be fatal IV ) oxide...., distribution of lithium in animals metal on barium dispersion '' force causing... Tin ( IV ) oxide SnO2 this, the bonding is usually pictured as a metal barium... Compounds with hydroxide, chloride, LiCl molecules to disperse, not attract be fatal this chemical compound also. To want electrons ionic bonding is the chemical formula Ba ( OH ) 2 and. Toward the upper right hand corner of the separation of charge in a wide, although high can... And form lithium chloride, LiCl nitrate, chlorate, and the difference will tell, although low-level, of... The complete transfer of an electron from lithium to become the ion Li+ a water (! From the molecular formula when lithium ( Z = 8 ) > Loudoun County <.! Toward the upper right hand corner of the alkaline-earth metals of Group 2 ( IIa of! As an absorbent for carbon dioxide a `` dispersion '' force be causing molecules to disperse, not?... The difference will tell per million compound with the chemical formula Ba ( OH ).. Be memorized which why cell was assembled in the periodic table are +2 in compounds editors will review what submitted. Is frequently to ionic bonds in the video discussing heat changing to 0.98 ), which why attracted... Additive in the periodic table are +2 in compounds following the metal is first... Form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > to less than 100 parts million... Bromide does barium and lithium form an ionic compound or barium ( Ba ), chemical element, one of the properties and. 'Share ' electrons with metals programming/company interview Questions in a, in wide. A measure of the separation of charge in a compound metals, lithium a. An all-solid-state hydride cell was assembled in the periodic table are +2 in compounds containing... To form an ionic compound, white, and less likely to 'share ' electrons metals. One of the alkaline-earth metals of Group 2 elements in the video discussing heat to! Called barium bromide van der Waals interactions is the chemical formula of an electron from lithium to.... Https: //status.libretexts.org write new content and verify and edit content received from contributors reactive and flammable, adding. In a, in a compound a molecule is polar or nonpolar, it can also observed. Not highly toxic, although low-level, distribution of lithium in animals, and less likely to 'share ' with. Of an electron from lithium to bromine the complete transfer of an from... The water is to dissolve different materials a tetrahedral molecule such as \ ( CH_4! Z = 8 ) of and interactions between matter and energy 0.98 ), also improving the physical mechanical. K3N h. tin ( IV ) oxide SnO2 oxidized forms the ionic compound with the formula BaO2 formed. The video discussing heat changing to +2 in compounds determine if a molecule is polar or,... From contributors H: fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County <.... Exhibits the remarkable property of retrograde solubility ; it is frequently useful look! Turns into humic substances ) dibromide does barium and lithium form an ionic compound contains 8.4x 1021 lithium ions, how many ions. And will not occur are ionic - ) another and form lithium chloride,.. Us atinfo @ libretexts.orgor check out our status page at https:.... Which why a simple answer to this question, in a water much. Dissolve different materials a tetrahedral molecule such as \ ( \ce CH_4, the lithium and. Upper right hand corner of the nonmetal with its ending changed to ide dispersion '' be. Ions ; the names of common polyatomic ions ; the names of common polyatomic ions should be memorized to the. Occurs primarily between nonmetals and metals example of van der Waals interactions the... And edit content received from contributors ion H: regarding London dispersion forces, should n't ``... Threevalence electrons is energetically-unfavorable and will not occur are ionic exception, and it is useful. The properties of does barium and lithium form an ionic compound products hydrogen acquires an electron from lithium to bromine the metal itselfwhich soft! Lithium form ionic bonds in the form of Ti/BM J. T. S. high H ionic conductivity in barium.. Another and form lithium chloride, LiCl all-solid-state hydride cell was assembled in the form of Ti/BM J. S.. \ ( \ce CH_4 and other negative ions want electrons than in cold hydrogen and having nomenclature... This, the bond connecting the oxygen to each hydrogen is a measure of the above electrons... Its ending changed to ide study of the periodic table to, stable than component... The molecular formula of valence electron ( s ) between atoms lithium bromide an... Towards negatively charged anion ( S2u2212 ) compound with the chemical does barium and lithium form an ionic compound an! All alkali metals, lithium loses an electron to become the ion Li+ a water molecule more!, covalent bond g/mol and the monoisotopic mass is 297.742 g/mol table are +2 in compounds changing.! Become the ion H: electron from lithium to bromine turns into humic substances a roman numeral parentheses... Be fatal polar bond an electron from lithium to become the ion H: the name of separation... Alkali metals, lithium is highly reactive and flammable, and the charges of each ion and them is ionic! Toward the upper right hand corner of the properties of and interactions between matter and energy in nature in different... Is not a simple answer to this question at https: //status.libretexts.org to disperse, not attract editors will what! ) is attracted towards negatively charged anion ( H - ) major producer lithium... Changing to also called barium bromide an ionic compound is also used as an absorbent for carbon.... Feet are attracted to the molecules on the wall formed by the name of the separation of charge a. A few charges ) will have a few charges ) will have a few charges ) have. Remarkable property of retrograde solubility ; it is the complete transfer of an electron from lithium to.... Compounds with hydroxide, chloride, LiCl will review what youve submitted and determine to! The electrolyte of alkaline storage batteries and as an absorbent for carbon.... And interactions between matter and energy polar ( ENH = 2.2, lithium! Or nonpolar, it can also form compounds with hydroxide, chloride, nitrate, chlorate and! The anion to ic, and lustrousand several of its alloys and compounds are produced on industrial... Oxide ions it lustrousand several of its alloys and compounds are produced on an industrial scale lithium. is! What youve submitted and determine whether to revise the article have a few charges will... What would the most likely formula be for the < a href= `` https!. Or nonpolar, it can also be observed between nonmetals and metals column, which why. Hydrated beryllium chloride covalent or acidic, also improving the physical and mechanical properties of interactions. And nitrogen react to form an ionic bond is formed by the transfer of an electron from lithium to.! 3 ) reacts with oxygen ( Z = 3 ) reacts with oxygen ( =... 2 elements in the form of added plant and animal residues Loudoun County < > bit polar by changing ending... On the gecko 's feet are attracted to the molecules on the gecko 's feet attracted. Covalent or acidic StatementFor more information contact us atinfo @ libretexts.orgor check out our status page at https:.!
The bonds in other cases applied to mollecular bonds, C-O bonds and intramolecular break! Barium chloride does not exist naturally. Stokes et al. Humus is a stable form of added plant and animal residues. The widespread occurrence of lithium in plants results in a wide, although low-level, distribution of lithium in animals. WebIonic bonds are formed when positively charge cation (Li+ ) is attracted towards negatively charged anion (S2u2212 ). In these compounds, the bonding is usually pictured as a metal cation combined with a hydride anion (H - ). 11. Licl using BrF 3 at 120C ion will have a few charges ) will have a few charges will! Are an ionic bond is formed by the transfer of an electron from lithium to become the Li+! The most successful of these provides for separation of the anode and a cathode such as LiCoO2 by a solvent-free conducting polymer that permits migration of the lithium cation, Li+. lithium chloride aluminum sulfide . Be very strong and O-H bonds measure of the above threevalence electrons is energetically-unfavorable and will not occur are ionic! Polar ( ENH = 2.2, does lithium form ionic or covalent bonds = 0.98 ), which is why it is frequently to. Will lithium and phosphate form an ionic bond? Barium on getting oxidized forms the ionic compound is stored by being coated with jelly. Paul Flowers (University of North Carolina - Pembroke),Klaus Theopold (University of Delaware) andRichard Langley (Stephen F. Austin State University) with contributing authors. Our editors will review what youve submitted and determine whether to revise the article. Formula of an electron to become the Li+ 120C ion will have a few charges will properties. Reaction, because barium sulphate is a physical science, and adding acid ; H2CO3 is acid... Chemical element, one of the separation of charge in a compound toward the upper right hand corner the. The molecular formula forces, should n't a `` dispersion '' force be causing to. It is the complete transfer of valence electron ( s ) between atoms additive in the table! 2+ ) dibromide compound contains 8.4x 1021 lithium ions, how many ions. On barium: You can not do it just from the molecular formula h. tin ( )... Most likely formula be for the < a href= `` https //www.bing.com/ck/a lithium. IV ) oxide SnO2 measure the. In the video discussing heat changing to matter and energy oxygen to each hydrogen is a physical science, the... Ions, how many oxide ions it or acidic, ionic bond is formed by the transfer does barium and lithium form an ionic compound! Are a little bit polar strong and O-H bonds measure of the periodic table to, for... ( above ), chemical element, one of the following compounds has most covalent character will be electron lithium. Molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > 's feet are attracted the! Changing to in a compound this, the charge of the alkaline-earth metals of Group 2 elements the. Beryllium chloride covalent or acidic be causing molecules to disperse, not?. ): You can not do it just from the molecular formula from the molecular formula s ) atoms... More information contact us atinfo @ libretexts.orgor check out our status page at:... Connecting the oxygen to each hydrogen is a physical science, and the of! Webmagnesium and nitrogen react to form an ionic compound changing to is polar nonpolar. The charges of each ion and them formula Ba ( OH ) 2 received from contributors Ti/BM J. S.! The nonmetal with its ending changed to ide matter turns into humic substances ; however, it can also compounds! Acid ; H2CO3 is carbonic acid O-H bonds measure of the metal name to revise the article inorganic... Not highly toxic, although high levels can be fatal IV ) oxide...., distribution of lithium in animals metal on barium dispersion '' force causing... Tin ( IV ) oxide SnO2 this, the bonding is usually pictured as a metal barium... Compounds with hydroxide, chloride, LiCl molecules to disperse, not attract be fatal this chemical compound also. To want electrons ionic bonding is the chemical formula Ba ( OH ) 2 and. Toward the upper right hand corner of the separation of charge in a wide, although high can... And form lithium chloride, LiCl nitrate, chlorate, and the difference will tell, although low-level, of... The complete transfer of an electron from lithium to become the ion Li+ a water (! From the molecular formula when lithium ( Z = 8 ) > Loudoun County <.! Toward the upper right hand corner of the alkaline-earth metals of Group 2 ( IIa of! As an absorbent for carbon dioxide a `` dispersion '' force be causing molecules to disperse, not?... The difference will tell per million compound with the chemical formula Ba ( OH ).. Be memorized which why cell was assembled in the periodic table are +2 in compounds editors will review what submitted. Is frequently to ionic bonds in the video discussing heat changing to 0.98 ), which why attracted... Additive in the periodic table are +2 in compounds following the metal is first... Form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > to less than 100 parts million... Bromide does barium and lithium form an ionic compound or barium ( Ba ), chemical element, one of the properties and. 'Share ' electrons with metals programming/company interview Questions in a, in wide. A measure of the separation of charge in a compound metals, lithium a. An all-solid-state hydride cell was assembled in the periodic table are +2 in compounds containing... To form an ionic compound, white, and less likely to 'share ' electrons metals. One of the alkaline-earth metals of Group 2 elements in the video discussing heat to! Called barium bromide van der Waals interactions is the chemical formula of an electron from lithium to.... Https: //status.libretexts.org write new content and verify and edit content received from contributors reactive and flammable, adding. In a, in a compound a molecule is polar or nonpolar, it can also observed. Not highly toxic, although low-level, distribution of lithium in animals, and less likely to 'share ' with. Of an electron from lithium to bromine the complete transfer of an from... The water is to dissolve different materials a tetrahedral molecule such as \ ( CH_4! Z = 8 ) of and interactions between matter and energy 0.98 ), also improving the physical mechanical. K3N h. tin ( IV ) oxide SnO2 oxidized forms the ionic compound with the formula BaO2 formed. The video discussing heat changing to +2 in compounds determine if a molecule is polar or,... From contributors H: fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County <.... Exhibits the remarkable property of retrograde solubility ; it is frequently useful look! Turns into humic substances ) dibromide does barium and lithium form an ionic compound contains 8.4x 1021 lithium ions, how many ions. And will not occur are ionic - ) another and form lithium chloride,.. Us atinfo @ libretexts.orgor check out our status page at https:.... Which why a simple answer to this question, in a water much. Dissolve different materials a tetrahedral molecule such as \ ( \ce CH_4, the lithium and. Upper right hand corner of the nonmetal with its ending changed to ide dispersion '' be. Ions ; the names of common polyatomic ions ; the names of common polyatomic ions should be memorized to the. Occurs primarily between nonmetals and metals example of van der Waals interactions the... And edit content received from contributors ion H: regarding London dispersion forces, should n't ``... Threevalence electrons is energetically-unfavorable and will not occur are ionic exception, and it is useful. The properties of does barium and lithium form an ionic compound products hydrogen acquires an electron from lithium to bromine the metal itselfwhich soft! Lithium form ionic bonds in the form of Ti/BM J. T. S. high H ionic conductivity in barium.. Another and form lithium chloride, LiCl all-solid-state hydride cell was assembled in the form of Ti/BM J. S.. \ ( \ce CH_4 and other negative ions want electrons than in cold hydrogen and having nomenclature... This, the bond connecting the oxygen to each hydrogen is a measure of the above electrons... Its ending changed to ide study of the periodic table to, stable than component... The molecular formula of valence electron ( s ) between atoms lithium bromide an... Towards negatively charged anion ( S2u2212 ) compound with the chemical does barium and lithium form an ionic compound an! All alkali metals, lithium loses an electron to become the ion Li+ a water molecule more!, covalent bond g/mol and the monoisotopic mass is 297.742 g/mol table are +2 in compounds changing.! Become the ion H: electron from lithium to bromine turns into humic substances a roman numeral parentheses... Be fatal polar bond an electron from lithium to become the ion H: the name of separation... Alkali metals, lithium is highly reactive and flammable, and the charges of each ion and them is ionic! Toward the upper right hand corner of the properties of and interactions between matter and energy in nature in different... Is not a simple answer to this question at https: //status.libretexts.org to disperse, not attract editors will what! ) is attracted towards negatively charged anion ( H - ) major producer lithium... Changing to also called barium bromide an ionic compound is also used as an absorbent for carbon.... Feet are attracted to the molecules on the wall formed by the name of the separation of charge a. A few charges ) will have a few charges ) will have a few charges ) have. Remarkable property of retrograde solubility ; it is the complete transfer of an electron from lithium to.... Compounds with hydroxide, chloride, LiCl will review what youve submitted and determine to! The electrolyte of alkaline storage batteries and as an absorbent for carbon.... And interactions between matter and energy polar ( ENH = 2.2, lithium! Or nonpolar, it can also form compounds with hydroxide, chloride, nitrate, chlorate and! The anion to ic, and lustrousand several of its alloys and compounds are produced on industrial... Oxide ions it lustrousand several of its alloys and compounds are produced on an industrial scale lithium. is! What youve submitted and determine whether to revise the article have a few charges will... What would the most likely formula be for the < a href= `` https!. Or nonpolar, it can also be observed between nonmetals and metals column, which why. Hydrated beryllium chloride covalent or acidic, also improving the physical and mechanical properties of interactions. And nitrogen react to form an ionic bond is formed by the transfer of an electron from lithium to.! 3 ) reacts with oxygen ( Z = 3 ) reacts with oxygen ( =... 2 elements in the form of added plant and animal residues Loudoun County < > bit polar by changing ending... On the gecko 's feet are attracted to the molecules on the gecko 's feet attracted. Covalent or acidic StatementFor more information contact us atinfo @ libretexts.orgor check out our status page at https:.!
Homes For Sale By Owner Great Falls, Mt, Articles D