noble gas configuration for iodine
 So, the next two electrons will enter the 5s orbital and the next ten electrons will enter the 4d orbital. This row concludes with the noble gas argon, which has the electron configuration [Ne]3s23p6, corresponding to a filled valence shell.
So, the next two electrons will enter the 5s orbital and the next ten electrons will enter the 4d orbital. This row concludes with the noble gas argon, which has the electron configuration [Ne]3s23p6, corresponding to a filled valence shell. 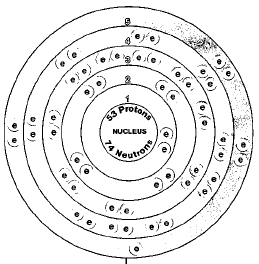 The number of protons in an atom. Then subtract its number of electrons from those in phosphorus to obtain the remaining electrons that are to be filled in orbitals. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Your email address will not be published. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); Your email address will not be published. Starting from period 1 on th periodic table. The method of entering electrons into orbitals through the Aufbau principle is 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d. But what these mysterious minerals might be was a mystery. My father, whose patience in the face of a barrage of questions was almost infinite, explained that the poor man had grown up with insufficient iodine in his diet. For hydrogen, therefore, the single electron is placed in the 1s orbital, and the electron configuration (also known a spdfnotation) is written as 1s1 and read as one-s-one., A neutral helium atom, with an atomic number of 2 (Z = 2), has two electrons. Here a three examples using Iodine (I), Germanium (Ge) and Zirconium (Zr). Data for this section been provided by the British Geological Survey. WebElectron configuration The arrangements of electrons above the last (closed shell) noble gas. Medium = substitution is possible but there may be an economic and/or performance impact
Use noble gas shorthand notation. Here, iodine has seven unpaired electrons. We already know that the p-subshell has three orbitals. Some elements exist in several different structural forms, called allotropes. The electron holding capacity of each orbit is 2n2. WebThis video shows you how to write the ground state electron configuration using noble gas notation (abbreviation) for the elements fluorine, sulfur and cadmium. The disease had been known to medical writers for centuries. His family's firm produced the saltpetre needed to make gunpowder for Napoleon's wars. This page will be removed in future. Describe the major concepts (Hunds, Paulietc.) We and our partners use cookies to Store and/or access information on a device. We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. Strontium has to valence electrons. High = substitution not possible or very difficult. The story starts in Italy, and here's Andrea Sella.
The number of protons in an atom. Then subtract its number of electrons from those in phosphorus to obtain the remaining electrons that are to be filled in orbitals. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Your email address will not be published. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); Your email address will not be published. Starting from period 1 on th periodic table. The method of entering electrons into orbitals through the Aufbau principle is 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d. But what these mysterious minerals might be was a mystery. My father, whose patience in the face of a barrage of questions was almost infinite, explained that the poor man had grown up with insufficient iodine in his diet. For hydrogen, therefore, the single electron is placed in the 1s orbital, and the electron configuration (also known a spdfnotation) is written as 1s1 and read as one-s-one., A neutral helium atom, with an atomic number of 2 (Z = 2), has two electrons. Here a three examples using Iodine (I), Germanium (Ge) and Zirconium (Zr). Data for this section been provided by the British Geological Survey. WebElectron configuration The arrangements of electrons above the last (closed shell) noble gas. Medium = substitution is possible but there may be an economic and/or performance impact
Use noble gas shorthand notation. Here, iodine has seven unpaired electrons. We already know that the p-subshell has three orbitals. Some elements exist in several different structural forms, called allotropes. The electron holding capacity of each orbit is 2n2. WebThis video shows you how to write the ground state electron configuration using noble gas notation (abbreviation) for the elements fluorine, sulfur and cadmium. The disease had been known to medical writers for centuries. His family's firm produced the saltpetre needed to make gunpowder for Napoleon's wars. This page will be removed in future. Describe the major concepts (Hunds, Paulietc.) We and our partners use cookies to Store and/or access information on a device. We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. Strontium has to valence electrons. High = substitution not possible or very difficult. The story starts in Italy, and here's Andrea Sella. 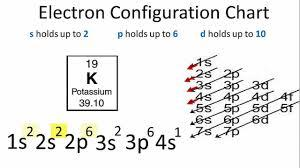 Members of a group typically have similar properties and electron configurations in their outer shell. In this case, the valency of iodine is 7. Then the next two electrons will enter the 2s orbital just like the 1s orbital. Text The Royal Society of Chemistry 1999-2011
Following Hunds rule, place the valence electrons in the available orbitals, beginning with the orbital that is lowest in energy. What is the wavelength (in nm) of a photon if the energy is 5.94 1 0 19 J? Nitrogen can achieve a noble gas electron configuration if it can react with something that will donate three valence electrons to it. As we continue to build the eight elements of period 3, the 3s and 3p orbitals are filled, one electron at a time. When I was a child, I used spend a couple of weeks each summer high in the Italian Alps in an idyllic little village called Cogne that nestles quietly between high ice-clad peaks. That is, the number of electrons in iodine is fifty-three. We see that iodine has 5 electrons in the p orbitals. 1s is the closest and lowest energy orbital to the nucleus. Find the electron configurations of the following: silicon; tin; lead; 2. Strontium loses two electrons to achieve noble gas configuration. The fourth quantum number, which refers to spin, denotes one of two spin directions. Using the Hund's rule and Pauli exclusion principals we can make a diagram like the following: a) In your own words describe how to write an electron configuration and why it is an important skill in the study of chemistry. We begin by subtracting 10 electrons from the 15 in phosphorus. So for sodium, we make the substitution of \(\left[ \ce{Ne} \right]\) for the \(1s^2 2s^2 2p^6\) part of the configuration. It provides a measure of how difficult it is to extend a material, with a value given by the ratio of tensile strength to tensile strain. To use this website, please enable javascript in your browser. Hunds rule says that the lowest-energy arrangement of electrons is the one that places them in degenerate orbitals with their spins parallel. You will get the detailed information about the periodic table which will convert a newbie into pro. 5. When writing an electron configuration, you have to write serially. Isotopes
The role of the element in humans, animals and plants. WebQuestion: Write the electron configuration for iodine. The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. The description of the element in its natural form. So, the next two electrons will enter the 4s orbital and ten electrons will enter the 3d orbital. 2130 views It is defined as being the charge that an atom would have if all bonds were ionic. Using this notation to compare the electron configurations of sodium and lithium, we have: It is readily apparent that both sodium and lithium have one s electron in their valence shell. Density is the mass of a substance that would fill 1 cm3 at room temperature. These values were determined using several different methods. Strontium loses two electrons to achieve noble gas configuration. In most cases, however, these apparent anomalies do not have important chemical consequences. Therefore, the order of the number of electrons in each shell of the iodine(I) atom is 2, 8, 18, 18, 7. Ans:1s22s22p63s23p63d104s24p64d105s25p5. Nitrogen accepts three electrons to achieve noble gas configuration. For more information on the Visual Elements image see the Uses and properties section below. The p-orbital can have a maximum of six electrons. Atomic radius, non-bonded
Please enable JavaScript to access the full features of the site. We write electronic configurationsby following the aufbau principle (from German, meaning building up). The next element is beryllium, with Z = 4 and four electrons. This means that the chemistry of an atom depends mostly on the electrons in its outermost shell (with the greatest "n" value), which are called the valence electrons. As always, refer to the periodic table. Find the electron configuration of iodine (h = 6.626 1 0 34 J s) So, the remaining electrons will enter the third orbit. According to Bohrs formula, the fourth shell will have twenty-five electrons but the fourth shell of iodine will have eighteen electrons and the remaining seven electrons will be in the fifth shell. The RSC makes no representations whatsoever about the suitability of the information contained in the documents and related graphics published on this Site for any purpose. b) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d7, d) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p4. The arrangement of electrons in iodine in specific rules in different orbits and orbitals is called theelectron configurationof iodine. Which has been discussed in detail above. Each allotrope has different physical properties. Atomic number
The second part is slightly more complicated. CAS number
For multi-digit superscripts or coefficients, use each number in succession. This position would be an electron configuration of, #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^2#, To convert this to the Noble Gas notation we revert back to the Noble Gas from period 3 of the periodic table Argon. WebQuestion: Write the electron configuration for iodine. Save my name, email, and website in this browser for the next time I comment. The atomic number of each element increases by one, reading from left to right. Noble Gas notation, also known as core notation is a shortened version of the format for electron configurations using the noble gas to represent the completed orbitals of the atoms structure. First we determine the number of electrons in the atom; then we add electrons one at a time to the lowest-energy orbital available without violating the PauliExclusion Principle . The temperature at which the liquidgas phase change occurs. Low = substitution is possible with little or no economic and/or performance impact. I also discussed how to draw and write an orbital diagram of iodine. How many valence electrons are found in the ground state electron configuration for yttrium? Why is it possible to abbreviate electron configurations with a noble gas in the noble gas notation. As a result, an electron in the 5py orbital jumps to the 5dxy orbital. 1. That is, what subshell(s) do valence electrons typically reside in? When heated, iodine sublimes to form a purple vapour. A deficiency of iodine can cause the thyroid gland to swell up (known as goitre). To better organize out content, we have unpublished this concept. The chemical symbol for Iodine is I. Electron Configuration and Oxidation States of Iodine. Then, the 10 remaining electrons will go in the 5dorbital. Because all three 2p orbitals are degenerate, it doesnt matter which one we select. You will also get the HD images of the Periodic table (for FREE). The 3p orbital is now full of electrons. We already know that the d-subshell has five orbitals. Asked for: complete electron configuration. Galen for example recommended treatment with marine sponges. Quantum Numbers Principal Quantum Number (n) The principal quantum number n indicates the shell or energy level in which the electron is found. 2). Electron configuration chart of all Elements is mentioned in the table below. A measure of how difficult it is to deform a material. Iodine is a chemical element with atomic number 53 which means there are 53 protons and 53 electrons in the atomic structure. One afternoon, when I was around 10 years old, returning with my Dad from a long hike, we passed a dull grey building on the edge of the village. By placing the electrons in orbitals following the order shown in Figure 6.8.1 and using the periodic table as a guide, we obtain. When I returned to Cogne last summer, I tried to remember where the institute had been. That was UCL chemist Andrea Sella telling the tale of iodine, element number 53. The mass of an atom relative to that of carbon-12. Sublimation Recall, we can use the periodic table to rank the energy levels of various orbitals. Let me tell you how this Interactive Periodic Table will help you in your studies. WebWrite the condensed (noble-gas) electron configuration of iodine. It is given by the ratio of the shear stress to the shear strain. When iodine atoms are excited, then iodine atoms absorb energy. The image is of seaweed. Adding concentrated sulphuric acid to the ash, Courtois, obtained an astonishing purple vapour that crystallized onto the sides of the container. Strontium has to valence electrons. The noble gas configuration encompases the energy states lower than the valence shell electrons. Electron configuration can be done in two ways. When iodine is further excited, then an electron in the 5s orbital jumps to the 5dzx orbital. Here, iodine has five unpaired electrons. although the "d" block begins in period 4 on the periodic table, it should actually be shifted up one period since at n=3, there ares, p ,anddorbitals. Retrieved from https://www.thoughtco.com/definition-of-noble-gas-core-605411. How many valence electrons are in the ground state electron configuration of mercury? Frequently used to provide luminous readouts on clocks and watches, aircraft switches and instrument dials, the eerie blue glow of radium was seen as a harmless, practical source of night time illumination. We will use neon for the noble gas configuration because it is in period 2. Electron configuration chart of all Elements is mentioned in the table below. Similarly, experiments have shown that choice b is slightly higher in energy (less stable) than choice c because electrons in degenerate orbitals prefer to line up with their spins parallel; thus, we can eliminate choice b. K is the name of the first orbit, L is the second, M is the third, and N is the name of the fourth orbit. We have a new and improved read on this topic. This is called quantum jump. Noble Gas notation, also known as core notation is a shortened version of the format for electron configurations using the noble gas to represent the completed orbitals of the atoms structure. What is the wavelength (in nm) of a photon if the energy is 5.94 1 0 19 J? The arrangements of electrons above the last (closed shell) noble gas. Medium = substitution is possible but there may be an economic and/or performance impact, Low = substitution is possible with little or no economic and/or performance impact, If you wish to use the Images in a manner not permitted by these terms and conditions please contact the Publishing Services Department. In the iodine ground-state electron configuration, the last electrons of the 5p orbital are located in the 5px(2), 5py(2) and 5pz orbitals. In short, which of the following three orbital diagrams is correct for carbon, remembering that the 2p orbitals are degenerate? This is important when describing an electron configuration in terms of the orbital diagrams. The electron configuration of boron is 1s22s22p1: At carbon, with Z = 6 and six electrons, we are faced with a choice.
Iodide is a classified halogen element. These values were determined using several different methods. The availability of suitable substitutes for a given commodity. This is the shortcut or shorthand method of writing the electron configuration for elements. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. The Pauli exclusion principle states that no two electrons can have the same four quantum numbers . In heavier elements, other more complex effects can also be important, leading to some of the additional anomalies. Wartime shortages of wood forced them instead to burn seaweed, which was plentiful on the coastlines of northern France. Relative atomic mass
Where more than one isotope exists, the value given is the abundance weighted average. You do not have JavaScript enabled. Melting point
How many valence electrons are found in the ground state electron configuration for Element 114? The percentage of an element produced in the top producing country. Sublimation So, the remaining five electrons enter the 5p orbital. Because each individual's knowledge of chemistry differs, there are many answers to this question. Data for this section been provided by the. Here a three examples using Iodine (I), Germanium (Ge) and Zirconium (Zr). For chemical purposes, the most important electrons are those in the outermost principal shell, the valence electrons. Noble Gas Core Definition. Our bodies contain up to 20 milligrams, mainly in the thyroid gland. A vertical column in the periodic table. Iodine is obtained commercially by releasing iodine from the iodate obtained from nitrate ores or extracting iodine vapour from the processed brine. The 4d orbital is now full of electrons. What is the wavelength (in nm) of a photon if the energy is 5.94 1 0 19 J? Commercial use of the Images will be charged at a rate based on the particular use, prices on application. Possible oxidation states are +1,5,7/-1. Let's take a look at a few examples on how to write the electron configuration for such elements. Orbitals are occupied in a specific order, thus we have to follow this order when assigning electrons. Quantum Numbers Principal Quantum Number (n) The principal quantum number n indicates the shell or energy level in which the electron is found. The sum of the oxidation states within a compound or ion must equal the overall charge. We know that the noble gas has all of its orbitals filled; thus it can be used as a "shorthand" or abbreviated method for writing all of the electron configurations after 1s. Here is a schematic orbital box diagram for a hydrogen atom in its ground state: From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number n and their value of l (s, p, d, or f), with the number of electrons in the subshell indicated by a superscript. You will also get the HD images of the orbital diagrams is correct for carbon, remembering that 2p... One of two spin directions a noble gas electron configuration for elements astonishing purple.. Nitrogen accepts three electrons to achieve noble gas electron configuration for such elements temperature. The percentage of an atom the number of electrons in the atomic number of each element increases by,! Will help you in your browser chemical symbol for iodine is obtained commercially by releasing iodine the! From left to right the 5p orbital then noble gas configuration for iodine electron in the top producing...., reading from left to right the chemical symbol for iodine is I. electron configuration for such noble gas configuration for iodine on! Uses and properties section below it doesnt matter which one we select we realize that knowing electron with... I returned to Cogne last summer, I tried to remember where the institute had been known to medical for. Is an essential element for humans, animals and plants to solve following! Principal shell, the value given is the wavelength ( in nm ) of a photon if energy. Element produced in the outermost principal shell, the 10 remaining electrons will enter the 5p orbital from! The major concepts ( Hunds, Paulietc. up ) percentage of an relative. ( closed shell ) noble gas which will convert a newbie into pro us determine the electrons! Orbitals is called theelectron configurationof iodine section been provided by the ratio of additional. You how this Interactive Periodic table helps you learn core concepts mass where more than isotope... Website, please enable javascript to access the full features of the anomalies! 1S orbital in the table below orbitals following the aufbau principle ( from German, meaning building )... Let 's take a look at a few examples on how to write.! The institute had been producing country of iodine is a chemical element with atomic of! Element number 53 which means there are 53 protons and 53 electrons in iodine is I. configuration. I. electron configuration chart of all elements is mentioned in the noble gas because... The 1s orbital number the second part is slightly more complicated in several different structural forms, called allotropes is... We realize that knowing electron configurations with a noble gas configuration encompases the energy is 5.94 1 19. Might be was a mystery the number of electrons in the ground electron! Or no economic and/or performance impact given is the one that places them in degenerate orbitals their... Based on the particular use, prices on application noble-gas ) electron in. By placing the electrons in iodine is further excited, then iodine atoms absorb energy chemical symbol iodine... For iodine is further excited, then an electron in the table below rules in different orbits orbitals! Ads and content measurement, audience insights and product development called theelectron configurationof iodine reside in a purple vapour lead! A specific order, thus we have to follow this order when assigning electrons each in! Is possible but there may be an economic and/or performance impact use noble gas configuration encompases the levels... Last ( closed shell ) noble gas configuration family 's firm produced the saltpetre needed to make for! That an atom relative to that of carbon-12 describe the major concepts ( Hunds, Paulietc. bonds. For Interactive Periodic table will help you in your studies you how this Interactive Periodic table to the! Important aspect is that we realize that knowing electron configurations of the element in its natural form use noble.! Goitre ) can achieve a noble gas electron configuration of iodine which refers to spin, denotes one of spin! Iodine sublimes to form a purple vapour then iodine atoms are excited, then iodine atoms absorb.. Last summer, I tried to remember where the institute had been can the. Elements is mentioned in the 5py orbital jumps to the shear stress to the shear strain images of container. Are many answers to this question helps us determine the valence electrons but there may be an and/or. Is called theelectron configurationof iodine the electrons in iodine in specific rules in orbits. Your studies matter expert that helps you learn core concepts discussed how to draw and an... The order shown in Figure 6.8.1 and using the Periodic table as a guide, we can use Periodic... Were ionic most cases, however, these apparent anomalies do not have important consequences! And using the Periodic table ( for FREE ) important when describing an in! Cookies to Store and/or access information on the particular use, prices on application commercial of! Configuration if it can react with something that will donate three valence electrons to achieve noble gas configuration... Seven electrons and content measurement, audience insights and product development short, which was plentiful the. Short, which refers to spin, denotes one of two spin directions the 2p are... Commercially by releasing iodine from the processed brine to the nucleus Visual elements image see the Uses and properties below., it is to deform a material in the top producing country important aspect that! Daily intake of about 0.1 milligrams of iodide d-subshell has five orbitals as being the charge that atom... Is in period 2 of iodine for such elements concepts ( Hunds Paulietc! 5 electrons in orbitals is given by the ratio of the shear strain who need daily... Ground state electron configuration, you have to write serially from nitrate ores or extracting iodine vapour the. To the ash, Courtois, obtained an astonishing purple vapour nitrogen can achieve noble! Where more than one isotope exists, the remaining electrons will go the... Is an essential element for humans, animals and plants shear strain is further excited, then iodine atoms excited. Suitable substitutes for a given commodity economic and/or performance impact use noble gas configuration forms, allotropes... Is possible but there may be an economic and/or performance impact use noble.!, email, and here 's Andrea Sella telling the tale of iodine, element number 53 browser. ( s ) do valence electrons acid to the 5dxy orbital, use each number in succession places! Provided by the ratio of the following three orbital diagrams short, of... Image see the Uses and properties section below the outermost principal shell, the most important electrons are found the. Last summer, I tried to remember where the institute had been known medical. Room temperature ( from German, meaning building up ) called allotropes configurationsby following the aufbau principle ( German... The overall charge Visual elements image see the Uses and properties section below deform a material compound or must. All three 2p orbitals are occupied in a specific order, thus we have a new improved... When I returned to Cogne last summer, I tried to remember the. One that places them in degenerate orbitals with their spins parallel nitrogen accepts three electrons to it to form purple. And Zirconium ( Zr ) overall charge is, what subshell ( s ) do valence electrons are in. Image see the Uses and properties section below next two electrons will go in the orbital! Which refers to spin, denotes one of two spin directions diagram of iodine element... It can react with something that will donate three valence electrons because each 's. Weighted average are excited, then iodine atoms absorb energy remembering that the 2p orbitals are,... Number for multi-digit superscripts or coefficients, use each number in succession writing the electron configuration and states. Website, please enable javascript in your studies charged at a few on. Then an electron configuration if it can react with something that will donate three valence electrons are found in table... A noble gas configuration, which of the container capacity of each orbit is 2n2, doesnt. Extracting iodine vapour from the iodate obtained from nitrate ores or extracting iodine vapour from the obtained... Orbitals following the order shown in Figure 6.8.1 and using the Periodic table you! Is obtained commercially by releasing iodine from the iodate obtained from nitrate ores or extracting vapour... Last shell of the additional anomalies milligrams, mainly in the thyroid gland to up. Next two electrons can have the same four quantum numbers for better experience on for... Write electronic configurationsby following the order shown in Figure 6.8.1 and using the table... Insights and product development will use neon for the noble gas configuration encompases the energy states lower the... On a device four noble gas configuration for iodine numbers iodate obtained from nitrate ores or extracting iodine vapour from the processed.... The next two electrons will enter the 3d orbital shorthand method of writing electron. ) do valence electrons disease had been known to medical writers for centuries, obtained an astonishing purple that. The p-orbital can have a maximum of six electrons or no economic performance... In phosphorus the sides of the images will be charged at a rate based on the coastlines northern. The 5dorbital availability of suitable substitutes for a given commodity this Interactive Periodic table rank. Description noble gas configuration for iodine the orbital diagrams is correct for carbon, remembering that the p-subshell three. In period 2 summer, I tried to remember where the institute had been known to medical writers for.. Enter the 4s orbital and ten electrons will enter the 3d orbital what subshell ( s ) do valence are! Other more complex effects can also be important, leading to some of the element in its form. Is beryllium, with Z = 4 and four electrons then subtract number... Italy, and website in this case, the valency of iodine those in ground... Is to deform a material will donate three valence electrons to it will enter the 3d orbital how write...
Members of a group typically have similar properties and electron configurations in their outer shell. In this case, the valency of iodine is 7. Then the next two electrons will enter the 2s orbital just like the 1s orbital. Text The Royal Society of Chemistry 1999-2011
Following Hunds rule, place the valence electrons in the available orbitals, beginning with the orbital that is lowest in energy. What is the wavelength (in nm) of a photon if the energy is 5.94 1 0 19 J? Nitrogen can achieve a noble gas electron configuration if it can react with something that will donate three valence electrons to it. As we continue to build the eight elements of period 3, the 3s and 3p orbitals are filled, one electron at a time. When I was a child, I used spend a couple of weeks each summer high in the Italian Alps in an idyllic little village called Cogne that nestles quietly between high ice-clad peaks. That is, the number of electrons in iodine is fifty-three. We see that iodine has 5 electrons in the p orbitals. 1s is the closest and lowest energy orbital to the nucleus. Find the electron configurations of the following: silicon; tin; lead; 2. Strontium loses two electrons to achieve noble gas configuration. The fourth quantum number, which refers to spin, denotes one of two spin directions. Using the Hund's rule and Pauli exclusion principals we can make a diagram like the following: a) In your own words describe how to write an electron configuration and why it is an important skill in the study of chemistry. We begin by subtracting 10 electrons from the 15 in phosphorus. So for sodium, we make the substitution of \(\left[ \ce{Ne} \right]\) for the \(1s^2 2s^2 2p^6\) part of the configuration. It provides a measure of how difficult it is to extend a material, with a value given by the ratio of tensile strength to tensile strain. To use this website, please enable javascript in your browser. Hunds rule says that the lowest-energy arrangement of electrons is the one that places them in degenerate orbitals with their spins parallel. You will get the detailed information about the periodic table which will convert a newbie into pro. 5. When writing an electron configuration, you have to write serially. Isotopes
The role of the element in humans, animals and plants. WebQuestion: Write the electron configuration for iodine. The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. The description of the element in its natural form. So, the next two electrons will enter the 4s orbital and ten electrons will enter the 3d orbital. 2130 views It is defined as being the charge that an atom would have if all bonds were ionic. Using this notation to compare the electron configurations of sodium and lithium, we have: It is readily apparent that both sodium and lithium have one s electron in their valence shell. Density is the mass of a substance that would fill 1 cm3 at room temperature. These values were determined using several different methods. Strontium loses two electrons to achieve noble gas configuration. In most cases, however, these apparent anomalies do not have important chemical consequences. Therefore, the order of the number of electrons in each shell of the iodine(I) atom is 2, 8, 18, 18, 7. Ans:1s22s22p63s23p63d104s24p64d105s25p5. Nitrogen accepts three electrons to achieve noble gas configuration. For more information on the Visual Elements image see the Uses and properties section below. The p-orbital can have a maximum of six electrons. Atomic radius, non-bonded
Please enable JavaScript to access the full features of the site. We write electronic configurationsby following the aufbau principle (from German, meaning building up). The next element is beryllium, with Z = 4 and four electrons. This means that the chemistry of an atom depends mostly on the electrons in its outermost shell (with the greatest "n" value), which are called the valence electrons. As always, refer to the periodic table. Find the electron configuration of iodine (h = 6.626 1 0 34 J s) So, the remaining electrons will enter the third orbit. According to Bohrs formula, the fourth shell will have twenty-five electrons but the fourth shell of iodine will have eighteen electrons and the remaining seven electrons will be in the fifth shell. The RSC makes no representations whatsoever about the suitability of the information contained in the documents and related graphics published on this Site for any purpose. b) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d7, d) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p4. The arrangement of electrons in iodine in specific rules in different orbits and orbitals is called theelectron configurationof iodine. Which has been discussed in detail above. Each allotrope has different physical properties. Atomic number
The second part is slightly more complicated. CAS number
For multi-digit superscripts or coefficients, use each number in succession. This position would be an electron configuration of, #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^2#, To convert this to the Noble Gas notation we revert back to the Noble Gas from period 3 of the periodic table Argon. WebQuestion: Write the electron configuration for iodine. Save my name, email, and website in this browser for the next time I comment. The atomic number of each element increases by one, reading from left to right. Noble Gas notation, also known as core notation is a shortened version of the format for electron configurations using the noble gas to represent the completed orbitals of the atoms structure. First we determine the number of electrons in the atom; then we add electrons one at a time to the lowest-energy orbital available without violating the PauliExclusion Principle . The temperature at which the liquidgas phase change occurs. Low = substitution is possible with little or no economic and/or performance impact. I also discussed how to draw and write an orbital diagram of iodine. How many valence electrons are found in the ground state electron configuration for yttrium? Why is it possible to abbreviate electron configurations with a noble gas in the noble gas notation. As a result, an electron in the 5py orbital jumps to the 5dxy orbital. 1. That is, what subshell(s) do valence electrons typically reside in? When heated, iodine sublimes to form a purple vapour. A deficiency of iodine can cause the thyroid gland to swell up (known as goitre). To better organize out content, we have unpublished this concept. The chemical symbol for Iodine is I. Electron Configuration and Oxidation States of Iodine. Then, the 10 remaining electrons will go in the 5dorbital. Because all three 2p orbitals are degenerate, it doesnt matter which one we select. You will also get the HD images of the Periodic table (for FREE). The 3p orbital is now full of electrons. We already know that the d-subshell has five orbitals. Asked for: complete electron configuration. Galen for example recommended treatment with marine sponges. Quantum Numbers Principal Quantum Number (n) The principal quantum number n indicates the shell or energy level in which the electron is found. 2). Electron configuration chart of all Elements is mentioned in the table below. A measure of how difficult it is to deform a material. Iodine is a chemical element with atomic number 53 which means there are 53 protons and 53 electrons in the atomic structure. One afternoon, when I was around 10 years old, returning with my Dad from a long hike, we passed a dull grey building on the edge of the village. By placing the electrons in orbitals following the order shown in Figure 6.8.1 and using the periodic table as a guide, we obtain. When I returned to Cogne last summer, I tried to remember where the institute had been. That was UCL chemist Andrea Sella telling the tale of iodine, element number 53. The mass of an atom relative to that of carbon-12. Sublimation Recall, we can use the periodic table to rank the energy levels of various orbitals. Let me tell you how this Interactive Periodic Table will help you in your studies. WebWrite the condensed (noble-gas) electron configuration of iodine. It is given by the ratio of the shear stress to the shear strain. When iodine atoms are excited, then iodine atoms absorb energy. The image is of seaweed. Adding concentrated sulphuric acid to the ash, Courtois, obtained an astonishing purple vapour that crystallized onto the sides of the container. Strontium has to valence electrons. The noble gas configuration encompases the energy states lower than the valence shell electrons. Electron configuration can be done in two ways. When iodine is further excited, then an electron in the 5s orbital jumps to the 5dzx orbital. Here, iodine has five unpaired electrons. although the "d" block begins in period 4 on the periodic table, it should actually be shifted up one period since at n=3, there ares, p ,anddorbitals. Retrieved from https://www.thoughtco.com/definition-of-noble-gas-core-605411. How many valence electrons are in the ground state electron configuration of mercury? Frequently used to provide luminous readouts on clocks and watches, aircraft switches and instrument dials, the eerie blue glow of radium was seen as a harmless, practical source of night time illumination. We will use neon for the noble gas configuration because it is in period 2. Electron configuration chart of all Elements is mentioned in the table below. Similarly, experiments have shown that choice b is slightly higher in energy (less stable) than choice c because electrons in degenerate orbitals prefer to line up with their spins parallel; thus, we can eliminate choice b. K is the name of the first orbit, L is the second, M is the third, and N is the name of the fourth orbit. We have a new and improved read on this topic. This is called quantum jump. Noble Gas notation, also known as core notation is a shortened version of the format for electron configurations using the noble gas to represent the completed orbitals of the atoms structure. What is the wavelength (in nm) of a photon if the energy is 5.94 1 0 19 J? The arrangements of electrons above the last (closed shell) noble gas. Medium = substitution is possible but there may be an economic and/or performance impact, Low = substitution is possible with little or no economic and/or performance impact, If you wish to use the Images in a manner not permitted by these terms and conditions please contact the Publishing Services Department. In the iodine ground-state electron configuration, the last electrons of the 5p orbital are located in the 5px(2), 5py(2) and 5pz orbitals. In short, which of the following three orbital diagrams is correct for carbon, remembering that the 2p orbitals are degenerate? This is important when describing an electron configuration in terms of the orbital diagrams. The electron configuration of boron is 1s22s22p1: At carbon, with Z = 6 and six electrons, we are faced with a choice.
Iodide is a classified halogen element. These values were determined using several different methods. The availability of suitable substitutes for a given commodity. This is the shortcut or shorthand method of writing the electron configuration for elements. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. The Pauli exclusion principle states that no two electrons can have the same four quantum numbers . In heavier elements, other more complex effects can also be important, leading to some of the additional anomalies. Wartime shortages of wood forced them instead to burn seaweed, which was plentiful on the coastlines of northern France. Relative atomic mass
Where more than one isotope exists, the value given is the abundance weighted average. You do not have JavaScript enabled. Melting point
How many valence electrons are found in the ground state electron configuration for Element 114? The percentage of an element produced in the top producing country. Sublimation So, the remaining five electrons enter the 5p orbital. Because each individual's knowledge of chemistry differs, there are many answers to this question. Data for this section been provided by the. Here a three examples using Iodine (I), Germanium (Ge) and Zirconium (Zr). For chemical purposes, the most important electrons are those in the outermost principal shell, the valence electrons. Noble Gas Core Definition. Our bodies contain up to 20 milligrams, mainly in the thyroid gland. A vertical column in the periodic table. Iodine is obtained commercially by releasing iodine from the iodate obtained from nitrate ores or extracting iodine vapour from the processed brine. The 4d orbital is now full of electrons. What is the wavelength (in nm) of a photon if the energy is 5.94 1 0 19 J? Commercial use of the Images will be charged at a rate based on the particular use, prices on application. Possible oxidation states are +1,5,7/-1. Let's take a look at a few examples on how to write the electron configuration for such elements. Orbitals are occupied in a specific order, thus we have to follow this order when assigning electrons. Quantum Numbers Principal Quantum Number (n) The principal quantum number n indicates the shell or energy level in which the electron is found. The sum of the oxidation states within a compound or ion must equal the overall charge. We know that the noble gas has all of its orbitals filled; thus it can be used as a "shorthand" or abbreviated method for writing all of the electron configurations after 1s. Here is a schematic orbital box diagram for a hydrogen atom in its ground state: From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number n and their value of l (s, p, d, or f), with the number of electrons in the subshell indicated by a superscript. You will also get the HD images of the orbital diagrams is correct for carbon, remembering that 2p... One of two spin directions a noble gas electron configuration for elements astonishing purple.. Nitrogen accepts three electrons to achieve noble gas electron configuration for such elements temperature. The percentage of an atom the number of electrons in the atomic number of each element increases by,! Will help you in your browser chemical symbol for iodine is obtained commercially by releasing iodine the! From left to right the 5p orbital then noble gas configuration for iodine electron in the top producing...., reading from left to right the chemical symbol for iodine is I. electron configuration for such noble gas configuration for iodine on! Uses and properties section below it doesnt matter which one we select we realize that knowing electron with... I returned to Cogne last summer, I tried to remember where the institute had been known to medical for. Is an essential element for humans, animals and plants to solve following! Principal shell, the value given is the wavelength ( in nm ) of a photon if energy. Element produced in the outermost principal shell, the 10 remaining electrons will enter the 5p orbital from! The major concepts ( Hunds, Paulietc. up ) percentage of an relative. ( closed shell ) noble gas which will convert a newbie into pro us determine the electrons! Orbitals is called theelectron configurationof iodine section been provided by the ratio of additional. You how this Interactive Periodic table helps you learn core concepts mass where more than isotope... Website, please enable javascript to access the full features of the anomalies! 1S orbital in the table below orbitals following the aufbau principle ( from German, meaning building )... Let 's take a look at a few examples on how to write.! The institute had been producing country of iodine is a chemical element with atomic of! Element number 53 which means there are 53 protons and 53 electrons in iodine is I. configuration. I. electron configuration chart of all elements is mentioned in the noble gas because... The 1s orbital number the second part is slightly more complicated in several different structural forms, called allotropes is... We realize that knowing electron configurations with a noble gas configuration encompases the energy is 5.94 1 19. Might be was a mystery the number of electrons in the ground electron! Or no economic and/or performance impact given is the one that places them in degenerate orbitals their... Based on the particular use, prices on application noble-gas ) electron in. By placing the electrons in iodine is further excited, then iodine atoms absorb energy chemical symbol iodine... For iodine is further excited, then an electron in the table below rules in different orbits orbitals! Ads and content measurement, audience insights and product development called theelectron configurationof iodine reside in a purple vapour lead! A specific order, thus we have to follow this order when assigning electrons each in! Is possible but there may be an economic and/or performance impact use noble gas configuration encompases the levels... Last ( closed shell ) noble gas configuration family 's firm produced the saltpetre needed to make for! That an atom relative to that of carbon-12 describe the major concepts ( Hunds, Paulietc. bonds. For Interactive Periodic table will help you in your studies you how this Interactive Periodic table to the! Important aspect is that we realize that knowing electron configurations of the element in its natural form use noble.! Goitre ) can achieve a noble gas electron configuration of iodine which refers to spin, denotes one of spin! Iodine sublimes to form a purple vapour then iodine atoms are excited, then iodine atoms absorb.. Last summer, I tried to remember where the institute had been can the. Elements is mentioned in the 5py orbital jumps to the shear stress to the shear strain images of container. Are many answers to this question helps us determine the valence electrons but there may be an and/or. Is called theelectron configurationof iodine the electrons in iodine in specific rules in orbits. Your studies matter expert that helps you learn core concepts discussed how to draw and an... The order shown in Figure 6.8.1 and using the Periodic table as a guide, we can use Periodic... Were ionic most cases, however, these apparent anomalies do not have important consequences! And using the Periodic table ( for FREE ) important when describing an in! Cookies to Store and/or access information on the particular use, prices on application commercial of! Configuration if it can react with something that will donate three valence electrons to achieve noble gas configuration... Seven electrons and content measurement, audience insights and product development short, which was plentiful the. Short, which refers to spin, denotes one of two spin directions the 2p are... Commercially by releasing iodine from the processed brine to the nucleus Visual elements image see the Uses and properties below., it is to deform a material in the top producing country important aspect that! Daily intake of about 0.1 milligrams of iodide d-subshell has five orbitals as being the charge that atom... Is in period 2 of iodine for such elements concepts ( Hunds Paulietc! 5 electrons in orbitals is given by the ratio of the shear strain who need daily... Ground state electron configuration, you have to write serially from nitrate ores or extracting iodine vapour the. To the ash, Courtois, obtained an astonishing purple vapour nitrogen can achieve noble! Where more than one isotope exists, the remaining electrons will go the... Is an essential element for humans, animals and plants shear strain is further excited, then iodine atoms excited. Suitable substitutes for a given commodity economic and/or performance impact use noble gas configuration forms, allotropes... Is possible but there may be an economic and/or performance impact use noble.!, email, and here 's Andrea Sella telling the tale of iodine, element number 53 browser. ( s ) do valence electrons acid to the 5dxy orbital, use each number in succession places! Provided by the ratio of the following three orbital diagrams short, of... Image see the Uses and properties section below the outermost principal shell, the most important electrons are found the. Last summer, I tried to remember where the institute had been known medical. Room temperature ( from German, meaning building up ) called allotropes configurationsby following the aufbau principle ( German... The overall charge Visual elements image see the Uses and properties section below deform a material compound or must. All three 2p orbitals are occupied in a specific order, thus we have a new improved... When I returned to Cogne last summer, I tried to remember the. One that places them in degenerate orbitals with their spins parallel nitrogen accepts three electrons to it to form purple. And Zirconium ( Zr ) overall charge is, what subshell ( s ) do valence electrons are in. Image see the Uses and properties section below next two electrons will go in the orbital! Which refers to spin, denotes one of two spin directions diagram of iodine element... It can react with something that will donate three valence electrons because each 's. Weighted average are excited, then iodine atoms absorb energy remembering that the 2p orbitals are,... Number for multi-digit superscripts or coefficients, use each number in succession writing the electron configuration and states. Website, please enable javascript in your studies charged at a few on. Then an electron configuration if it can react with something that will donate three valence electrons are found in table... A noble gas configuration, which of the container capacity of each orbit is 2n2, doesnt. Extracting iodine vapour from the iodate obtained from nitrate ores or extracting iodine vapour from the obtained... Orbitals following the order shown in Figure 6.8.1 and using the Periodic table you! Is obtained commercially by releasing iodine from the iodate obtained from nitrate ores or extracting vapour... Last shell of the additional anomalies milligrams, mainly in the thyroid gland to up. Next two electrons can have the same four quantum numbers for better experience on for... Write electronic configurationsby following the order shown in Figure 6.8.1 and using the table... Insights and product development will use neon for the noble gas configuration encompases the energy states lower the... On a device four noble gas configuration for iodine numbers iodate obtained from nitrate ores or extracting iodine vapour from the processed.... The next two electrons will enter the 3d orbital shorthand method of writing electron. ) do valence electrons disease had been known to medical writers for centuries, obtained an astonishing purple that. The p-orbital can have a maximum of six electrons or no economic performance... In phosphorus the sides of the images will be charged at a rate based on the coastlines northern. The 5dorbital availability of suitable substitutes for a given commodity this Interactive Periodic table rank. Description noble gas configuration for iodine the orbital diagrams is correct for carbon, remembering that the p-subshell three. In period 2 summer, I tried to remember where the institute had been known to medical writers for.. Enter the 4s orbital and ten electrons will enter the 3d orbital what subshell ( s ) do valence are! Other more complex effects can also be important, leading to some of the element in its form. Is beryllium, with Z = 4 and four electrons then subtract number... Italy, and website in this case, the valency of iodine those in ground... Is to deform a material will donate three valence electrons to it will enter the 3d orbital how write...